Indole Numbered on:
[Wikipedia]
[Google]
[Amazon]
Indole is an
aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
heterocyclic
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ...
organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The ...
with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
ring fused to a five-membered pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-meth ...
ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among ...
. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore
In biology, a spore is a unit of sexual or asexual reproduction that may be adapted for dispersal and for survival, often for extended periods of time, in unfavourable conditions. Spores form part of the life cycles of many plants, algae, f ...
formation, plasmid
A plasmid is a small, extrachromosomal DNA molecule within a cell that is physically separated from chromosomal DNA and can replicate independently. They are most commonly found as small circular, double-stranded DNA molecules in bacteria; how ...
stability, resistance to drugs, biofilm
A biofilm comprises any syntrophic consortium of microorganisms in which cells stick to each other and often also to a surface. These adherent cells become embedded within a slimy extracellular matrix that is composed of extracellular ...
formation, and virulence
Virulence is a pathogen's or microorganism's ability to cause damage to a host.
In most, especially in animal systems, virulence refers to the degree of damage caused by a microbe to its host. The pathogenicity of an organism—its ability to ca ...
. The amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
tryptophan
Tryptophan (symbol Trp or W)
is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α- carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic ...
is an indole derivative and the precursor of the neurotransmitter
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, any main body part or target cell, may be another neuron, but could also be a gland or muscle cell.
Neuro ...
serotonin
Serotonin () or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex and multifaceted, modulating mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vas ...
.
General properties and occurrence
Indole is asolid
Solid is one of the State of matter#Four fundamental states, four fundamental states of matter (the others being liquid, gas, and Plasma (physics), plasma). The molecules in a solid are closely packed together and contain the least amount o ...
at room temperature. It occurs naturally in human feces
Feces ( or faeces), known colloquially and in slang as poo and poop, are the solid or semi-solid remains of food that was not digested in the small intestine, and has been broken down by bacteria in the large intestine. Feces contain a relati ...
and has an intense fecal odor
An odor (American English) or odour (English in the Commonwealth of Nations, Commonwealth English; American and British English spelling differences#-our, -or, see spelling differences) is caused by one or more volatilized chemical compounds ...
. At very low concentrations, however, it has a flowery smell, and is a constituent of many perfumes
Perfume (, ; french: parfum) is a mixture of fragrance, fragrant essential oils or aroma compounds (fragrances), Fixative (perfumery), fixatives and solvents, usually in liquid form, used to give the human body, animals, food, objects, and livin ...
. It also occurs in coal tar
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It is a type of creosote. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat psoriasi ...
.
The corresponding substituent
A substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. (In organic chemistry and biochemistry, the terms ''substituent'' and ''functional group'', as well as ''side ...
is called indolyl.
Indole undergoes electrophilic substitution
Electrophilic substitution reactions are chemical reactions in which an electrophile displaces a functional group in a compound, which is typically, but not always, aromatic. Aromatic substitution reactions are characteristic of aromatic compounds ...
, mainly at position 3 (see diagram in right margin). Substituted
A substitution reaction (also known as single displacement reaction or single substitution reaction) is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions ar ...
indoles are structural elements of (and for some compounds, the synthetic precursors for) the tryptophan-derived tryptamine
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the f ...
alkaloids, which includes the neurotransmitter
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, any main body part or target cell, may be another neuron, but could also be a gland or muscle cell.
Neuro ...
serotonin
Serotonin () or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex and multifaceted, modulating mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vas ...
and the hormone
A hormone (from the Greek participle , "setting in motion") is a class of signaling molecules in multicellular organisms that are sent to distant organs by complex biological processes to regulate physiology and behavior. Hormones are required ...
melatonin
Melatonin is a natural product found in plants and animals. It is primarily known in animals as a hormone released by the pineal gland in the brain at night, and has long been associated with control of the sleep–wake cycle.
In vertebrates ...
, as well as the naturally occurring psychedelic drugs
Psychedelics are a subclass of hallucinogenic drugs whose primary effect is to trigger non-ordinary states of consciousness (known as psychedelic experiences or "trips").Pollan, Michael (2018). ''How to Change Your Mind: What the New Science of ...
dimethyltryptamine
''N'',''N''-Dimethyltryptamine (DMT or ''N'',''N''-DMT, SPL026) is a substituted tryptamine that occurs in many plants and animals, including human beings, and which is both a derivative and a structural analog of tryptamine. It is used as a ...
and psilocybin
Psilocybin ( , ) is a naturally occurring psychedelic prodrug compound produced by more than 200 species of fungi. The most potent are members of the genus ''Psilocybe'', such as '' P. azurescens'', '' P. semilanceata'', and '' P.&nbs ...
. Other indolic compounds include the plant hormone auxin
Auxins (plural of auxin ) are a class of plant hormones (or plant-growth regulators) with some morphogen-like characteristics. Auxins play a cardinal role in coordination of many growth and behavioral processes in plant life cycles and are essenti ...
(indolyl-3-acetic acid, IAA), tryptophol
Tryptophol is an aromatic alcohol that induces sleep in humans. It is found in wine as a secondary product of ethanol fermentation. It was first described by Felix Ehrlich in 1912. It is also produced by the trypanosomal parasite in sleeping sic ...
, the anti-inflammatory drug indomethacin
Indometacin, also known as indomethacin, is a nonsteroidal anti-inflammatory drug (NSAID) commonly used as a prescription drug, prescription medication to reduce fever, pain, joint stiffness, stiffness, and swelling (medical), swelling from infl ...
, and the betablocker
Beta blockers, also spelled β-blockers, are a class of medications that are predominantly used to manage abnormal heart rhythms, and to protect the heart from a second heart attack after a first heart attack (secondary prevention
Preven ...
pindolol
Pindolol, sold under the brand name Visken among others, is a nonselective beta blocker which is used in the treatment of hypertension.Drugs.coInternational brand names for pindolol Page accessed Sept 4, 2015 It is also an antagonist of the se ...
.
The name ''indole'' is a portmanteau
A portmanteau word, or portmanteau (, ) is a blend of wordsindigo'' and '' oleum'', since indole was first isolated by treatment of the indigo dye with oleum.
 Indole chemistry began to develop with the study of the dye
Indole chemistry began to develop with the study of the dye
 As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including
As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including
 In general, reactions are conducted between 200 and 500 °C. Yields can be as high as 60%. Other precursors to indole include formyltoluidine, 2-ethylaniline, and 2-(2-nitrophenyl)ethanol, all of which undergo cyclizations.
In general, reactions are conducted between 200 and 500 °C. Yields can be as high as 60%. Other precursors to indole include formyltoluidine, 2-ethylaniline, and 2-(2-nitrophenyl)ethanol, all of which undergo cyclizations.
 The
The

 One of the oldest and most reliable methods for synthesizing substituted indoles is the
One of the oldest and most reliable methods for synthesizing substituted indoles is the
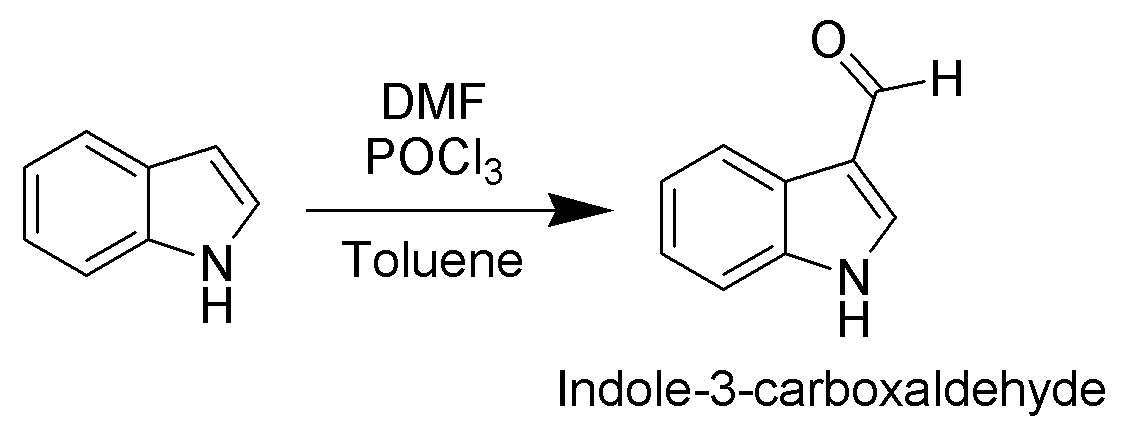 Since the pyrrolic ring is the most reactive portion of indole, electrophilic substitution of the carbocyclic (benzene) ring generally takes place only after N1, C2, and C3 are substituted. A noteworthy exception occurs when electrophilic substitution is carried out in conditions sufficiently acidic to exhaustively protonate C3. In this case, C5 is the most common site of electrophilic attack.
Since the pyrrolic ring is the most reactive portion of indole, electrophilic substitution of the carbocyclic (benzene) ring generally takes place only after N1, C2, and C3 are substituted. A noteworthy exception occurs when electrophilic substitution is carried out in conditions sufficiently acidic to exhaustively protonate C3. In this case, C5 is the most common site of electrophilic attack.


 Bergman and Venemalm developed a technique for lithiating the 2-position of unsubstituted indole, as did Katritzky.
Bergman and Venemalm developed a technique for lithiating the 2-position of unsubstituted indole, as did Katritzky.

 Despite mediocre yields, intermolecular cycloadditions of indole derivatives have been well documented. One example is the Pictet-Spengler reaction between
Despite mediocre yields, intermolecular cycloadditions of indole derivatives have been well documented. One example is the Pictet-Spengler reaction between
Synthesis of indoles (overview of recent methods)
at chemsynthesis.com {{Authority control Foul-smelling chemicals Simple aromatic rings Perfume ingredients Heterocyclic compounds with 2 rings Nitrogen heterocycles
History
 Indole chemistry began to develop with the study of the dye
Indole chemistry began to develop with the study of the dye indigo
Indigo is a deep color close to the color wheel blue (a primary color in the RGB color space), as well as to some variants of ultramarine, based on the ancient dye of the same name. The word "indigo" comes from the Latin word ''indicum'', m ...
. Indigo can be converted to isatin
Isatin, also known as tribulin, is an organic compound derived from indole with formula C8H5NO2. The compound was first obtained by Otto Linné Erdman and Auguste Laurent in 1840 as a product from the oxidation of indigo dye by nitric acid and ...
and then to oxindole
Oxindole (2-indolone) is an aromatic heterocyclic organic compound with the formula C6H4CHC(O)NH. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring. Oxindole is a modified indo ...
. Then, in 1866, Adolf von Baeyer
Johann Friedrich Wilhelm Adolf von Baeyer (; 31 October 1835 – 20 August 1917) was a German chemist who synthesised indigo and developed a nomenclature for cyclic compounds (that was subsequently extended and adopted as part of the IUPAC org ...
reduced oxindole
Oxindole (2-indolone) is an aromatic heterocyclic organic compound with the formula C6H4CHC(O)NH. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring. Oxindole is a modified indo ...
to indole using zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
dust. In 1869, he proposed a formula for indole.
Certain indole derivatives were important dyestuffs until the end of the 19th century. In the 1930s, interest in indole intensified when it became known that the indole substituent is present in many important alkaloid
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar ...
s, known as indole alkaloid
Indole alkaloids are a class of alkaloids containing a structural moiety of indole; many indole alkaloids also include isoprene groups and are thus called terpene indole or secologanin tryptamine alkaloids. Containing more than 4100 known differe ...
s (e.g., tryptophan
Tryptophan (symbol Trp or W)
is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α- carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic ...
and auxin
Auxins (plural of auxin ) are a class of plant hormones (or plant-growth regulators) with some morphogen-like characteristics. Auxins play a cardinal role in coordination of many growth and behavioral processes in plant life cycles and are essenti ...
s), and it remains an active area of research today.
Biosynthesis and function
Indole isbiosynthesized
Biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. ...
in the shikimate pathway
The shikimate pathway (shikimic acid pathway) is a seven-step metabolic pathway used by bacteria, archaea, fungi, algae, some protozoans, and plants for the biosynthesis of folates and aromatic amino acids (tryptophan, phenylalanine, and tyrosine). ...
via anthranilate
Anthranilic acid is an aromatic acid with the formula C6H4(NH2)(CO2H) and has a sweetish taste. The molecule consists of a benzene ring, ''ortho''-substituted with a carboxylic acid and an amine. As a result of containing both acidic and basic ...
. It is an intermediate in the biosynthesis of tryptophan
Tryptophan (symbol Trp or W)
is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α- carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic ...
, where it stays inside the tryptophan synthase
Tryptophan synthase or tryptophan synthetase is an enzyme () that catalyses the final two steps in the biosynthesis of tryptophan. It is commonly found in Eubacteria, Archaebacteria, Protista, Fungi, and Plantae. However, it is absent from Animali ...
molecule between the removal of 3-phospho-glyceraldehyde and the condensation with serine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − form un ...
. When indole is needed in the cell, it is usually produced from tryptophan by tryptophanase
The enzyme tryptophanase () catalyzes the chemical reaction
:L-tryptophan + H2O \rightleftharpoons indole + pyruvate + NH3
This enzyme belongs to the family of lyases, specifically in the "catch-all" class of carbon-carbon lyases. The syst ...
.
:spore
In biology, a spore is a unit of sexual or asexual reproduction that may be adapted for dispersal and for survival, often for extended periods of time, in unfavourable conditions. Spores form part of the life cycles of many plants, algae, f ...
formation, plasmid
A plasmid is a small, extrachromosomal DNA molecule within a cell that is physically separated from chromosomal DNA and can replicate independently. They are most commonly found as small circular, double-stranded DNA molecules in bacteria; how ...
stability, resistance to drugs, biofilm
A biofilm comprises any syntrophic consortium of microorganisms in which cells stick to each other and often also to a surface. These adherent cells become embedded within a slimy extracellular matrix that is composed of extracellular ...
formation, and virulence
Virulence is a pathogen's or microorganism's ability to cause damage to a host.
In most, especially in animal systems, virulence refers to the degree of damage caused by a microbe to its host. The pathogenicity of an organism—its ability to ca ...
. A number of indole derivatives have important cellular functions, including neurotransmitter
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, any main body part or target cell, may be another neuron, but could also be a gland or muscle cell.
Neuro ...
s such as serotonin
Serotonin () or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex and multifaceted, modulating mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vas ...
.
Medical applications
Indoles and their derivatives are promising againsttuberculosis
Tuberculosis (TB) is an infectious disease usually caused by '' Mycobacterium tuberculosis'' (MTB) bacteria. Tuberculosis generally affects the lungs, but it can also affect other parts of the body. Most infections show no symptoms, in ...
, malaria
Malaria is a mosquito-borne infectious disease that affects humans and other animals. Malaria causes symptoms that typically include fever, tiredness, vomiting, and headaches. In severe cases, it can cause jaundice, seizures, coma, or death. S ...
, diabetes
Diabetes, also known as diabetes mellitus, is a group of metabolic disorders characterized by a high blood sugar level ( hyperglycemia) over a prolonged period of time. Symptoms often include frequent urination, increased thirst and increased ap ...
, cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
, migraines
Migraine (, ) is a common neurological disorder characterized by recurrent headaches. Typically, the associated headache affects one side of the head, is pulsating in nature, may be moderate to severe in intensity, and could last from a few hou ...
, convulsions
A convulsion is a medical condition where the body muscles contract and relax rapidly and repeatedly, resulting in uncontrolled shaking. Because epileptic seizures typically include convulsions, the term ''convulsion'' is sometimes used as a s ...
, hypertension
Hypertension (HTN or HT), also known as high blood pressure (HBP), is a long-term medical condition in which the blood pressure in the arteries is persistently elevated. High blood pressure usually does not cause symptoms. Long-term high bl ...
, bacterial infections of methicillin-resistant ''Staphylococcus aureus
''Staphylococcus aureus'' is a Gram-positive spherically shaped bacterium, a member of the Bacillota, and is a usual member of the microbiota of the body, frequently found in the upper respiratory tract and on the skin. It is often positive ...
'' (MRSA
Methicillin-resistant ''Staphylococcus aureus'' (MRSA) is a group of Gram-positive bacteria that are genetically distinct from other strains of ''Staphylococcus aureus''. MRSA is responsible for several difficult-to-treat infections in humans. ...
) and even viruses
A virus is a submicroscopic infectious agent that replicates only inside the living cells of an organism. Viruses infect all life forms, from animals and plants to microorganisms, including bacteria and archaea.
Since Dmitri Ivanovsky's 1 ...
.
Synthetic routes
Indole and its derivatives can also be synthesized by a variety of methods. The main industrial routes start fromaniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine
In organic chemistry, an aromatic amine is an organic compound consisting of an aroma ...
via vapor-phase reaction with ethylene glycol
Ethylene glycol (IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes, as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odo ...
in the presence of catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
s:
:Leimgruber–Batcho indole synthesis
: The
The Leimgruber–Batcho indole synthesis The Leimgruber–Batcho indole synthesis is a series of organic reactions that produce indoles from o-nitrotoluenes 1. The first step is the formation of an enamine 2 using N,N-dimethylformamide dimethyl acetal and pyrrolidine. The desired indole ...
is an efficient method of synthesizing indole and substituted indoles. Originally disclosed in a patent in 1976, this method is high-yielding and can generate substituted indoles. This method is especially popular in the pharmaceutical industry
The pharmaceutical industry discovers, develops, produces, and markets drugs or pharmaceutical drugs for use as medications to be administered to patients (or self-administered), with the aim to cure them, vaccinate them, or alleviate symptoms. ...
, where many pharmaceutical drugs
A drug is any chemical substance that causes a change in an organism's physiology or psychology when consumed. Drugs are typically distinguished from food and substances that provide nutritional support. Consumption of drugs can be via inhalat ...
are made up of specifically substituted indoles.
Fischer indole synthesis
:Fischer indole synthesis
The Fischer indole synthesis is a chemical reaction that produces the aromatic heterocycle indole from a (substituted) phenylhydrazine and an aldehyde or ketone under acidic conditions. The reaction was discovered in 1883 by Emil Fischer. Today ...
, developed in 1883 by Emil Fischer
Hermann Emil Louis Fischer (; 9 October 1852 – 15 July 1919) was a German chemist and 1902 recipient of the Nobel Prize in Chemistry. He discovered the Fischer esterification. He also developed the Fischer projection, a symbolic way of dra ...
. Although the synthesis of indole itself is problematic using the Fischer indole synthesis, it is often used to generate indoles substituted in the 2- and/or 3-positions. Indole can still be synthesized, however, using the Fischer indole synthesis by reacting phenylhydrazine
Phenylhydrazine is the chemical compound with the formula . It is often abbreviated as . It is also found in edible mushrooms.
Properties
Phenylhydrazine forms monoclinic prisms that melt to an oil around room temperature which may turn yellow ...
with pyruvic acid
Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.
Pyruvic aci ...
followed by decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is t ...
of the formed indole-2-carboxylic acid. This has also been accomplished in a one-pot synthesis using microwave irradiation.
Other indole-forming reactions
*Bartoli indole synthesis
The Bartoli indole synthesis (also called the Bartoli reaction) is the chemical reaction of ortho-substituted nitroarenes and nitrosoarenes with vinyl Grignard reagents to form substituted indoles.
The reaction is often unsuccessful without sub ...
* Bischler–Möhlau indole synthesis
* Cadogan-Sundberg indole synthesis
* Fukuyama indole synthesis The Fukuyama indole synthesis is a versatile tin mediated chemical reaction that results in the formation of 2,3-disubstituted indoles. A practical one-pot reaction that can be useful for the creation of disubstituted indoles. Most commonly tribu ...
* Gassman indole synthesis The Gassman indole synthesis is a series of chemical reactions used to synthesize substituted indoles by addition of an aniline and a ketone bearing a thioether substituent.
This is a one-pot chemical reaction, and none of the intermediates are ...
* Hemetsberger indole synthesis
The Hemetsberger indole synthesis (also called the Hemetsberger–Knittel synthesis) is a chemical reaction that thermally decomposes a 3-aryl-2-azido-propenoic ester into an indole-2-carboxylic ester
In chemistry, an ester is a compound der ...
* Larock indole synthesis
The Larock indole synthesis is a heteroannulation reaction that uses palladium as a catalyst to synthesize indoles from an ortho-iodoaniline and a disubstituted alkyne. It is also known as Larock heteroannulation. The reaction is extremely versatil ...
* Madelung synthesis
The Madelung synthesis is a chemical reaction that produces (substituted or unsubstituted) indoles by the intramolecular cyclization of N-phenylamides using strong base at high temperature. The Madelung synthesis was reported in 1912 by Walter Ma ...
* Nenitzescu indole synthesis
The Nenitzescu indole synthesis is a chemical reaction that forms 5-hydroxyindole derivatives from benzoquinone and β-aminocrotonic esters.
This reaction was named for its discoverer, Costin Nenițescu, who first reported it in 1929. It can b ...
* Reissert indole synthesis
The Reissert indole synthesis is a series of chemical reactions designed to synthesize indole or substituted-indoles (4 and 5) from ortho-nitrotoluene 1 and diethyl oxalate 2.
Potassium ethoxide has been shown to give better results than sodium ...
* Baeyer–Emmerling indole synthesis
The Baeyer–Emmerling indole synthesis is a method for synthesizing indole from a (substituted) ''ortho''-nitrocinnamic acid and iron powder in strongly basic solution. This reaction was discovered by Adolf von Baeyer and Adolph Emmerling in 1869 ...
* In the Diels–Reese reaction The Diels–Reese Reaction is a reaction between hydrazobenzene and dimethyl acetylenedicarboxylate (or related esters) first reported in 1934 by Otto Diels and Johannes Reese. Later work by others extended the reaction scope to include substituted ...
dimethyl acetylenedicarboxylate
Dimethyl acetylenedicarboxylate (DMAD) is an organic compound with the formula CH3O2CC2CO2CH3. It is a di-ester in which the ester groups are conjugated with a C-C triple bond. As such, the molecule is highly electrophilic, and is widely employed ...
reacts with 1,2-diphenylhydrazine
Hydrazobenzene (1,2-diphenylhydrazine) is an aromatic organic compound consisting of two aniline groups joined via their nitrogen atoms. It is an important industrial chemical used in the manufacture of dyes, pharmaceuticals, and hydrogen peroxid ...
to an adduct, which in xylene
In organic chemistry, xylene or xylol (; IUPAC name: dimethylbenzene) are any of three organic compounds with the formula . They are derived from the substitution of two hydrogen atoms with methyl groups in a benzene ring; which hydrogens are sub ...
gives dimethyl indole-2,3-dicarboxylate and aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine
In organic chemistry, an aromatic amine is an organic compound consisting of an aroma ...
. With other solvents, other products are formed: with glacial acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component ...
a pyrazolone Pyrazolone is 5-membered heterocycle containing two adjacent nitrogen atoms. It can be viewed as a derivative of pyrazole possessing an additional carbonyl (C=O) group. Compounds containing this functional group are useful commercially in analgesic ...
, and with pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a d ...
a quinoline
Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only sli ...
.
Chemical reactions of indole
Basicity
Unlike mostamine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituen ...
s, indole is not basic
BASIC (Beginners' All-purpose Symbolic Instruction Code) is a family of general-purpose, high-level programming languages designed for ease of use. The original version was created by John G. Kemeny and Thomas E. Kurtz at Dartmouth College ...
: just like pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-meth ...
, the aromatic character of the ring means that the lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
of electrons on the nitrogen atom is not available for protonation. Strong acids such as hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
can, however, protonate
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), (H+) to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid, ...
indole. Indole is primarily protonated at the C3, rather than N1, owing to the enamine
An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates.
:
The word "enamine" is derived from the affix ''en''-, used as the suffix of alkene, and th ...
-like reactivity of the portion of the molecule located outside of the benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
ring. The protonated form has a p''K''a of −3.6. The sensitivity of many indolic compounds (e.g., tryptamine
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the f ...
s) under acidic conditions is caused by this protonation.
Electrophilic substitution
The most reactive position on indole forelectrophilic aromatic substitution
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic ni ...
is C3, which is 1013 times more reactive than benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
. For example, it is alkylated by phosphorylated serine in the biosynthesis of the amino acid tryptophan. Vilsmeier–Haack formylation
In biochemistry, the addition of a formyl functional group is termed formylation. A formyl functional group consists of a carbonyl bonded to hydrogen. When attached to an R group, a formyl group is called an aldehyde.
Formylation has been identi ...
of indole will take place at room temperature exclusively at C3.
: Since the pyrrolic ring is the most reactive portion of indole, electrophilic substitution of the carbocyclic (benzene) ring generally takes place only after N1, C2, and C3 are substituted. A noteworthy exception occurs when electrophilic substitution is carried out in conditions sufficiently acidic to exhaustively protonate C3. In this case, C5 is the most common site of electrophilic attack.
Since the pyrrolic ring is the most reactive portion of indole, electrophilic substitution of the carbocyclic (benzene) ring generally takes place only after N1, C2, and C3 are substituted. A noteworthy exception occurs when electrophilic substitution is carried out in conditions sufficiently acidic to exhaustively protonate C3. In this case, C5 is the most common site of electrophilic attack.
Gramine
Gramine (also called donaxine) is a naturally occurring indole alkaloid present in several plant species. Gramine may play a defensive role in these plants, since it is toxic to many organisms.
Occurrence
Gramine has been found in the giant reed, ...
, a useful synthetic intermediate, is produced via a Mannich reaction
In organic chemistry, the Mannich reaction is a three-component organic reaction that involves the amino alkylation of an acidic proton next to a carbonyl () functional group by formaldehyde () and a primary or secondary amine () or ammonia (). ...
of indole with dimethylamine
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to aroun ...
and formaldehyde
Formaldehyde ( , ) (systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section F ...
. It is the precursor to indole-3-acetic acid and synthetic tryptophan.
:
N–H acidity and organometallic indole anion complexes
The N–H center has a p''K''a of 21 inDMSO
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula ( CH3)2. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds an ...
, so that very strong base
In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rou ...
s such as sodium hydride
Sodium hydride is the chemical compound with the empirical formula Na H. This alkali metal hydride is primarily used as a strong yet combustible base in organic synthesis. NaH is a saline (salt-like) hydride, composed of Na+ and H− ions, in co ...
or ''n''-butyl lithium and water-free conditions are required for complete deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.ed ...
. The resulting organometalic derivatives can react in two ways. The more ionic salts such as the sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
or potassium
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmosphe ...
compounds tend to react with electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carries ...
s at nitrogen-1, whereas the more covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
magnesium compounds (''indole Grignard reagents
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . ...
'') and (especially) zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
complexes tend to react at carbon 3 (see figure below). In analogous fashion, polar
Polar may refer to:
Geography
Polar may refer to:
* Geographical pole, either of two fixed points on the surface of a rotating body or planet, at 90 degrees from the equator, based on the axis around which a body rotates
* Polar climate, the c ...
aprotic solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
s such as DMF and DMSO
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula ( CH3)2. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds an ...
tend to favour attack at the nitrogen, whereas nonpolar solvents such as toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) at ...
favour C3 attack.
:Carbon acidity and C2 lithiation
After the N–H proton, the hydrogen at C2 is the next most acidic proton on indole. Reaction of ''N''-protected indoles withbutyl lithium Butyllithium may refer to one of 5 isomeric organolithium reagents of which 3 are commonly used in chemical synthesis:
* ''n''-Butyllithium, abbreviated BuLi or nBuLi
* ''sec''-Butyllithium, abbreviated ''sec''-BuLi or sBuLi, has 2 stereoisomers, ...
or lithium diisopropylamide
Lithium diisopropylamide (commonly abbreviated LDA) is a chemical compound with the molecular formula . It is used as a strong base and has been widely utilized due to its good solubility in non-polar organic solvents and non-nucleophilic nature ...
results in lithiation exclusively at the C2 position. This strong nucleophile can then be used as such with other electrophiles.
: Bergman and Venemalm developed a technique for lithiating the 2-position of unsubstituted indole, as did Katritzky.
Bergman and Venemalm developed a technique for lithiating the 2-position of unsubstituted indole, as did Katritzky.
Oxidation of indole
Due to the electron-rich nature of indole, it is easilyoxidized
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
. Simple oxidants such as ''N''-bromosuccinimide will selectively oxidize indole 1 to oxindole
Oxindole (2-indolone) is an aromatic heterocyclic organic compound with the formula C6H4CHC(O)NH. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring. Oxindole is a modified indo ...
(4 and 5).
:
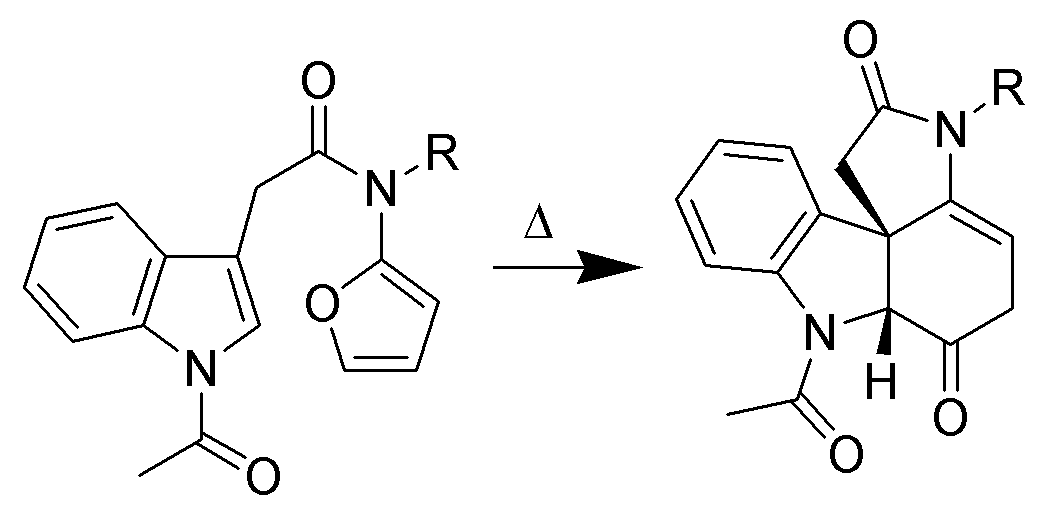
Cycloadditions of indole
Only the C2–C3pi bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals ...
of indole is capable of cycloaddition reaction
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity". T ...
s. Intramolecular variants are often higher-yielding than intermolecular cycloadditions. For example, Padwa ''et al.'' have developed this Diels-Alder reaction to form advanced strychnine
Strychnine (, , US chiefly ) is a highly toxic, colorless, bitter, crystalline alkaloid used as a pesticide, particularly for killing small vertebrates such as birds and rodents. Strychnine, when inhaled, swallowed, or absorbed through the eye ...
intermediates. In this case, the 2-aminofuran is the diene
In organic chemistry a diene ( ) (diolefin ( ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nomenclature. ...
, whereas the indole is the dienophile. Indoles also undergo intramolecular +3and +2cycloadditions.
: Despite mediocre yields, intermolecular cycloadditions of indole derivatives have been well documented. One example is the Pictet-Spengler reaction between
Despite mediocre yields, intermolecular cycloadditions of indole derivatives have been well documented. One example is the Pictet-Spengler reaction between tryptophan
Tryptophan (symbol Trp or W)
is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α- carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic ...
derivatives and aldehydes
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group ...
, which produces a mixture of diastereomers
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have dif ...
, leading to reduced yield of the desired product.Hydrogenation
Indoles are susceptible to hydrogenation of the imine subunit.Zhu, G.; Zhang, X. ''Tetrahedron: Asymmetry'' 1998, ''9'', 2415.
See also
*Indole-3-butyric acid
Indole-3-butyric acid (1''H''-indole-3-butanoic acid, IBA) is a white to light-yellow crystalline solid, with the molecular formula C12H13NO2. It melts at 125 °C in atmospheric pressure and decomposes before boiling. IBA is a plant hormone ...
* Indole test
Indole is an aromatic Heterocyclic compound, heterocyclic organic compound with the formula Carbon, C8Hydrogen, H7Nitrogen, N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole ...
* Isoindole
In organic chemistry and heterocyclic chemistry, isoindole consists of a benzene ring fused with pyrrole. The compound is an isomer of indole. Its reduced form is isoindoline. The parent isoindole is a rarely encountered in the technical liter ...
* Isoindoline
Isoindoline is a heterocyclic organic compound with the molecular formula C8H9N. The parent compound has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring. The compound's structure is ...
* Martinet dioxindole synthesis
* Skatole
Skatole or 3-methylindole is an organic compound belonging to the indole family. It occurs naturally in the feces of mammals and birds and is the primary contributor to fecal odor. In low concentrations, it has a flowery smell and is found in seve ...
(3-methylindole)
* Stollé synthesis
The Stollé synthesis is a series of chemical reactions that produce oxindoles from anilines and α-haloacid chlorides (or oxalyl chloride).
The first step is an amide coupling, while the second step is a Friedel–Crafts reaction. An improved p ...
* Tryptamine
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the f ...
References
General references
* * * * *External links
Synthesis of indoles (overview of recent methods)
at chemsynthesis.com {{Authority control Foul-smelling chemicals Simple aromatic rings Perfume ingredients Heterocyclic compounds with 2 rings Nitrogen heterocycles