|
Tryptamine
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the first one being the heterocyclic nitrogen). The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others. Tryptamine has been shown to activate trace amine-associated receptors expressed in the mammalian brain, and regulates the activity of dopaminergic, serotonergic and glutamatergic systems. In the human gut, symbiotic bacteria convert dietary tryptophan to tryptamine, which activates 5-HT4 receptors and regulates gastrointestinal motility. Multiple tryptamine-derived drugs have been developed to treat migraines, while trace amine-associated receptors are being explored as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Naturally Occurring Tryptamines
Substituted tryptamines, or serotonin analogues, are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino group, amino (NH2) group via an ethyl (−CH2–CH2−) side chain, sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms. Well-known tryptamines include serotonin, an important neurotransmitter, and melatonin, a hormone involved in regulating the sleep-wake cycle. Tryptamine alkaloids are found in fungi, plants and animals; and sometimes used by humans for the neurological or psychotropic effects of the substance. Prominent examples of tryptamine alkaloids include psilocybin (from "psilocybin mushrooms") and dimethyltryptamine, DMT. In South America, dimethyltryptamine is obtained from numerous plant sources, like chacruna, and it is often used in ayahuas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituted Tryptamine
Substituted tryptamines, or serotonin analogues, are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino group, amino (NH2) group via an ethyl (−CH2–CH2−) side chain, sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms. Well-known tryptamines include serotonin, an important neurotransmitter, and melatonin, a hormone involved in regulating the sleep-wake cycle. Tryptamine alkaloids are found in fungi, plants and animals; and sometimes used by humans for the neurological or psychotropic effects of the substance. Prominent examples of tryptamine alkaloids include psilocybin (from "psilocybin mushrooms") and dimethyltryptamine, DMT. In South America, dimethyltryptamine is obtained from numerous plant sources, like chacruna, and it is often used in ayahuas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
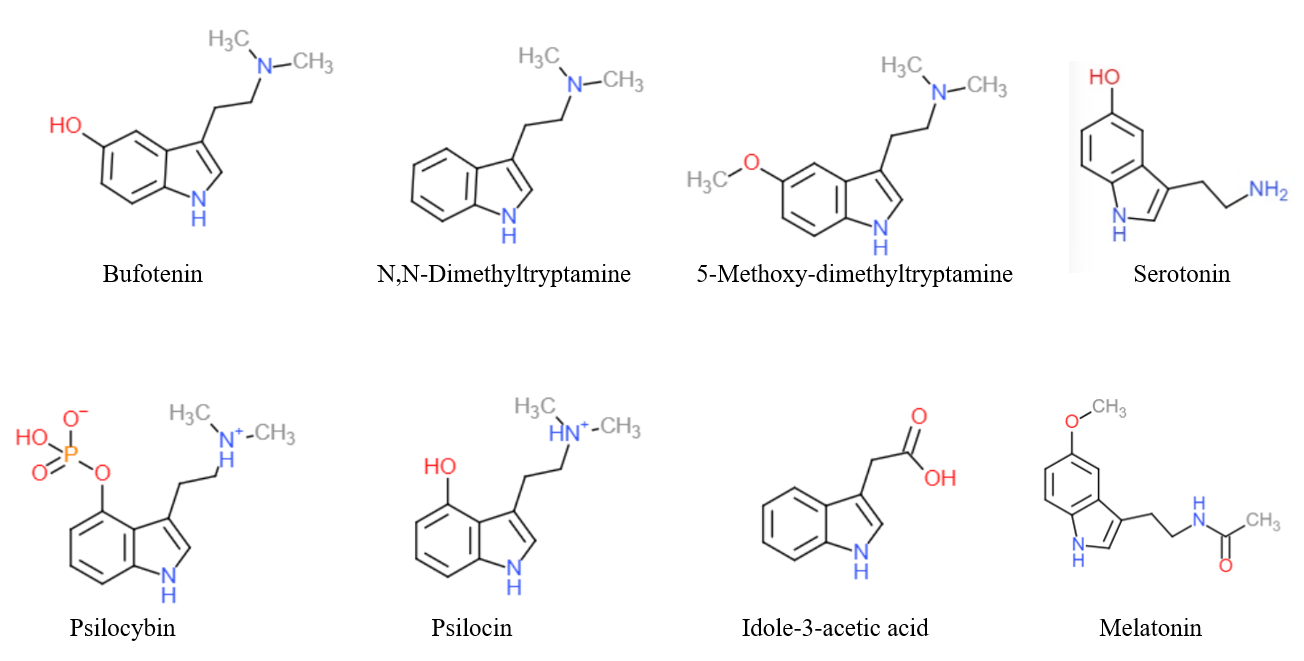
N,N-Dimethyltryptamine
''N'',''N''-Dimethyltryptamine (DMT or ''N'',''N''-DMT, SPL026) is a substituted tryptamine that occurs in many plants and animals, including human beings, and which is both a derivative and a structural analog of tryptamine. It is used as a psychedelic drug and prepared by various cultures for ritual purposes as an entheogen. DMT has a rapid onset, intense effects, and a relatively short duration of action. For those reasons, DMT was known as the "business trip" during the 1960s in the United States, as a user could access the full depth of a psychedelic experience in considerably less time than with other substances such as LSD or psilocybin mushrooms. DMT can be inhaled, ingested, or injected and its effects depend on the dose, as well as the mode of administration. When inhaled or injected, the effects last a short period of time: about five to 15 minutes. Effects can last three hours or more when orally ingested along with a monoamine oxidase inhibitor (MAOI), such as t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Psychoactive Plants
This is a list of plant species that, when consumed by humans, are known or suspected to produce psychoactive effects: changes in nervous system function that alter perception, mood, consciousness, cognition or behavior. Many of these plants are used intentionally as psychoactive drugs, for medicinal, religious, and/or recreational purposes. Some have been used ritually as entheogens for millennia. The plants are listed according to the specific psychoactive chemical substances they contain; many contain multiple known psychoactive compounds. Cannabinoids Species of the genus ''Cannabis'', known colloquially as marijuana, including ''Cannabis sativa'' and ''Cannabis indica'', is a popular psychoactive plant that is often used medically and recreationally. The principal psychoactive substance in ''Cannabis'', tetrahydrocannabinol (THC), contains no nitrogen, unlike many (but not all) other psychoactive substances and is not an indole, tryptamine, phenethylamine, anticholine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tryptamine Alkaloids 2
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the first one being the heterocyclic nitrogen). The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others. Tryptamine has been shown to activate trace amine-associated receptors expressed in the mammalian brain, and regulates the activity of dopaminergic, serotonergic and glutamatergic systems. In the human gut, symbiotic bacteria convert dietary tryptophan to tryptamine, which activates 5-HT4 receptors and regulates gastrointestinal motility. Multiple tryptamine-derived drugs have been developed to treat migraines, while trace amine-associated receptors are being explored as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonin
Serotonin () or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex and multifaceted, modulating mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vasoconstriction. Approximately 90% of the serotonin that the body produces is in the intestinal tract. Biochemically, the indoleamine molecule derives from the amino acid tryptophan, via the (rate-limiting) hydroxylation of the 5 position on the ring (forming the intermediate 5-hydroxytryptophan), and then decarboxylation to produce serotonin. Serotonin is primarily found in the enteric nervous system located in the gastrointestinal tract (GI tract). However, it is also produced in the central nervous system (CNS), specifically in the raphe nuclei located in the brainstem, Merkel cells located in the skin, pulmonary neuroendocrine cells and taste receptor cells in the tongue. Additionally, serotonin is stored in blood platelets and is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Psilocybin
Psilocybin ( , ) is a naturally occurring psychedelic prodrug compound produced by more than 200 species of fungi. The most potent are members of the genus ''Psilocybe'', such as '' P. azurescens'', '' P. semilanceata'', and '' P. cyanescens'', but psilocybin has also been isolated from about a dozen other genera. Psilocybin is itself biologically inactive but is quickly converted by the body to psilocin, which has mind-altering effects similar, in some aspects, to those of LSD, mescaline, and DMT. In general, the effects include euphoria, visual and mental hallucinations, changes in perception, a distorted sense of time, and perceived spiritual experiences. It can also cause adverse reactions such as nausea and panic attacks. Imagery found on prehistoric murals and rock paintings of modern-day Spain and Algeria suggests that human usage of psilocybin mushrooms predates recorded history. In Mesoamerica, the mushrooms had long been consumed in spiritual and div ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Psilocin
Psilocin (also known as 4-HO-DMT, 4-hydroxy DMT, psilocine, psilocyn, or psilotsin) is a substituted tryptamine alkaloid and a serotonergic psychedelic substance. It is present in most psychedelic mushrooms together with its phosphorylated counterpart psilocybin. Psilocin is a Schedule I drug under the Convention on Psychotropic Substances. Acting on the 5-HT2A receptors, psilocin modulates the production and reuptake of serotonin. The mind-altering effects of psilocin are highly variable and subjective and resemble those of LSD and DMT. Chemistry Psilocin and its phosphorylated cousin, psilocybin, were first isolated and named in 1958 by Swiss chemist Albert Hofmann. Hofmann obtained the chemicals from laboratory-grown specimens of the entheogenic mushroom ''Psilocybe mexicana''. Hofmann also succeeded in finding synthetic routes to these chemicals. Psilocin can be obtained by dephosphorylation of natural psilocybin under strongly acidic or under alkaline conditions (hyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bufotenin
Bufotenin (5-HO-DMT, bufotenine) is a tryptamine derivative - more specifically, a DMT derivative - related to the neurotransmitter serotonin. It is an alkaloid found in some species of toads (especially the skin), mushrooms and plants. The name bufotenin originates from the toad genus ''Bufo'', which includes several species of psychoactive toads, most notably ''Incilius alvarius'', that secrete bufotoxins from their parotoid glands. Bufotenin is similar in chemical structure to the psychedelics psilocin (4-HO-DMT), 5-MeO-DMT, and DMT, chemicals which also occur in some of the same fungus, plant, and animal species as bufotenin. Nomenclature Bufotenin (bufotenine) is also known by the chemical names 5-hydroxy-''N'',''N''-dimethyltryptamine (5-HO-DMT), ''N'',''N''-dimethyl-5-hydroxytryptamine, dimethyl serotonin, and mappine. History Bufotenin was isolated from toad skin, and named by the Austrian chemist Handovsky at the University of Prague during World War I. The struct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT4 Receptor
5-Hydroxytryptamine receptor 4 is a protein that in humans is encoded by the ''HTR4'' gene. Function This gene is a member of the family of human serotonin receptors, which are G protein-coupled receptors that stimulate cAMP production in response to serotonin (5-hydroxytryptamine). The gene product is a glycosylated transmembrane protein that functions in both the peripheral and central nervous system to modulate the release of various neurotransmitters. Multiple transcript variants encoding proteins with distinct C-terminal sequences have been described, but the full-length nature of some transcript variants has not been determined. Location The receptor is located in the alimentary tract, urinary bladder, heart and adrenal gland as well as the central nervous system (CNS). In the CNS the receptor appears in the putamen, caudate nucleus, nucleus accumbens, globus pallidus, and substantia nigra, and to a lesser extent in the neocortex, raphe, pontine nuclei, and some area ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melatonin
Melatonin is a natural product found in plants and animals. It is primarily known in animals as a hormone released by the pineal gland in the brain at night, and has long been associated with control of the sleep–wake cycle. In vertebrates, melatonin is involved in synchronizing circadian rhythms, including sleep–wake timing and blood pressure regulation, and in control of seasonal rhythmicity including reproduction, fattening, moulting and hibernation. Many of its effects are through activation of the melatonin receptors, while others are due to its role as an antioxidant. In plants, it functions to defend against oxidative stress. It is also present in various foods. Melatonin was discovered in 1958. In addition to its role as a natural hormone, melatonin is used as a dietary supplement and medication in the treatment of sleep disorders such as insomnia and circadian rhythm sleep disorders; for information on melatonin as a supplement and medication, see the melatoni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indole
Indole is an aromatic heterocyclic organic compound with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin. General properties and occurrence Indole is a solid at room temperature. It occurs naturally in human feces and has an intense fecal odor. At very low concentrations, however, it has a flowery smell, and is a constituent of many perfumes. It also occurs in coal tar. The corresponding substituent is called indolyl. Indole undergoes electrophilic substitution, mainly at position 3 (see diagra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |