|
Simple Aromatic Rings
Simple aromatic rings, also known as simple arenes or simple aromatics, are aromatic organic compounds that consist only of a conjugated planar ring system. Many simple aromatic rings have trivial names. They are usually found as substructures of more complex molecules ("substituted aromatics"). Typical simple aromatic compounds are benzene, indole, and pyridine. Simple aromatic rings can be heterocyclic if they contain non-carbon ring atoms, for example, oxygen, nitrogen, or sulfur. They can be monocyclic as in benzene, bicyclic as in naphthalene, or polycyclic as in anthracene. Simple monocyclic aromatic rings are usually five-membered rings like pyrrole or six-membered rings like pyridine. Fused/condensed aromatic rings consist of monocyclic rings that share their connecting bonds. Heterocyclic aromatic rings The nitrogen (N)-containing aromatic rings can be separated into basic aromatic rings that are easily protonated, and form aromatic cations and salts (e.g., pyridini ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to saturated compounds having single bonds, and other geometric or connective non-cyclic arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability. The term ''aromaticity'' with this meaning is historically related to the concept of having an aroma, but is a distinct property from that meaning. Since the most common aromatic compounds are derivatives of benzene (an aromatic hydrocarbon common in petroleum and its distillates), the word ''aromatic'' occasionally refers informally to benzene derivatives, and so it was first defined. Nevertheless, many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
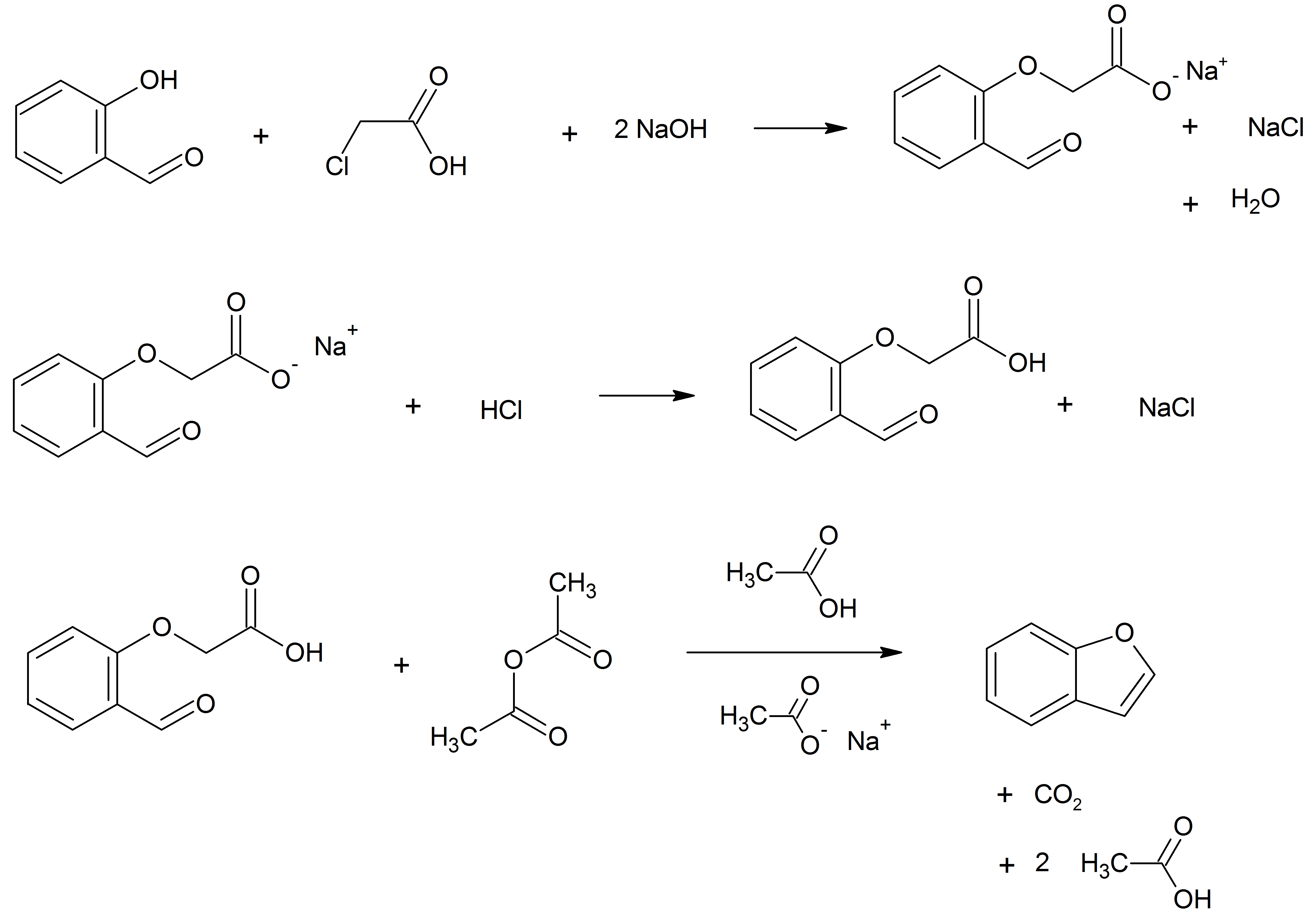
Benzofuran Structure
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the "parent" of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation. *Perkin rearrangement, where a coumarin is reacted with a hydroxide: : *Diels–Alder reaction of nitro vinyl furans with various dienophiles: : Diels–Alder reaction yielding a substituted benzofuran, 450px * Cycloisomerization of alkyne ortho-substituted phenols: : Benzofurans via Cycloisomerization, 400px Related compou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzo C Thiophen
Benzodiazepines (BZD, BDZ, BZs), sometimes called "benzos", are a class of depressant drugs whose core chemical structure is the fusion of a benzene ring and a diazepine ring. They are prescribed to treat conditions such as anxiety disorders, insomnia, and seizures. The first benzodiazepine, chlordiazepoxide (Librium), was discovered accidentally by Leo Sternbach in 1955 and was made available in 1960 by Hoffmann–La Roche, who soon followed with diazepam (Valium) in 1963. By 1977, benzodiazepines were the most prescribed medications globally; the introduction of selective serotonin reuptake inhibitors (SSRIs), among other factors, decreased rates of prescription, but they remain frequently used worldwide. Benzodiazepines are depressants that enhance the effect of the neurotransmitter gamma-aminobutyric acid (GABA) at the GABAA receptor, resulting in sedative, hypnotic ( sleep-inducing), anxiolytic (anti-anxiety), anticonvulsant, and muscle relaxant properties. High doses of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzothiophene
Benzothiophene is an aromatic organic compound with a molecular formula C8H6S and an odor similar to naphthalene (mothballs). It occurs naturally as a constituent of petroleum-related deposits such as lignite tar. Benzothiophene has no household use. In addition to benzo hiophene, a second isomer is known: benzo hiophene. Benzothiophene finds use in research as a starting material for the synthesis of larger, usually bioactive structures. It is found within the chemical structures of pharmaceutical drugs such as raloxifene, zileuton, and sertaconazole, and also BTCP. It is also used in the manufacturing of dyes such as thioindigo. Synthesis Most syntheses of benzothiophene create substituted benzothiophenes as a precursor to further reactions. An example is the reaction of an alkyne-substituted 2-bromobenzene with either sodium sulphide or potassium sulphide to form benzothiophene with an alkyl substitution at position 2. Thiourea Thiourea () is an organosulfur co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzothiophene Structure
Benzothiophene is an aromatic organic compound with a molecular formula C8H6S and an odor similar to naphthalene (mothballs). It occurs naturally as a constituent of petroleum-related deposits such as lignite tar. Benzothiophene has no household use. In addition to benzo hiophene, a second isomer is known: benzo hiophene. Benzothiophene finds use in research as a starting material for the synthesis of larger, usually bioactive structures. It is found within the chemical structures of pharmaceutical drugs such as , , and |
Thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reactions, it resembles benzene. Compounds analogous to thiophene include furan (C4H4O), selenophene (C4H4Se) and pyrrole (C4H4NH), which each vary by the heteroatom in the ring. Isolation and occurrence Thiophene was discovered as a contaminant in benzene. It was observed that isatin (an indole) forms a blue dye if it is mixed with sulfuric acid and crude benzene. The formation of the blue indophenin had long been believed to be a reaction of benzene itself. Viktor Meyer was able to isolate thiophene as the actual substance responsible for this reaction. Thiophene and especially its derivatives occur in petroleum, sometimes in concentrations up to 1–3%. The thiophenic content of oil and coal is removed via the hydrodesulfurization (HDS) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiophene Structure
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reactions, it resembles benzene. Compounds analogous to thiophene include furan (C4H4O), selenophene (C4H4Se) and pyrrole (C4H4NH), which each vary by the heteroatom in the ring. Isolation and occurrence Thiophene was discovered as a contaminant in benzene. It was observed that isatin (an indole) forms a blue dye if it is mixed with sulfuric acid and crude benzene. The formation of the blue indophenin had long been believed to be a reaction of benzene itself. Viktor Meyer was able to isolate thiophene as the actual substance responsible for this reaction. Thiophene and especially its derivatives occur in petroleum, sometimes in concentrations up to 1–3%. The thiophenic content of oil and coal is removed via the hydrodesulfurization (HDS) pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoindole
In organic chemistry and heterocyclic chemistry, isoindole consists of a benzene ring fused with pyrrole. The compound is an isomer of indole. Its reduced form is isoindoline. The parent isoindole is a rarely encountered in the technical literature, but substituted derivatives are useful commercially and occur naturally. Isoindoles units occur in phthalocyanines, an important family of dyes. Some alkaloids containing isoindole have been isolated and characterized.Heugebaert, Thomas S. A.; Roman, Bart I.; Stevens, Christian V. "Synthesis of isoindoles and related iso-condensed heteroaromatic pyrroles" Chemical Society Reviews 2012, volume 41, pp. 5626-5640. Synthesis The parent isoindole was prepared by flash vacuum pyrolysis of an N-substituted isoindoline. N-Substituted isoindoles, which are easier to handle, can be prepared by dehydration of isoindoline-N-oxides. They also arise by myriad other methods, e.g., starting from xylylene dibromide (C6H4(CH2Br)2). Structure and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indole
Indole is an aromatic heterocyclic organic compound with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin. General properties and occurrence Indole is a solid at room temperature. It occurs naturally in human feces and has an intense fecal odor. At very low concentrations, however, it has a flowery smell, and is a constituent of many perfumes. It also occurs in coal tar. The corresponding substituent is called indolyl. Indole undergoes electrophilic substitution, mainly at position 3 (see diagra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indol2
Indole is an aromatic heterocyclic organic compound with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin. General properties and occurrence Indole is a solid at room temperature. It occurs naturally in human feces and has an intense fecal odor. At very low concentrations, however, it has a flowery smell, and is a constituent of many perfumes. It also occurs in coal tar. The corresponding substituent is called indolyl. Indole undergoes electrophilic substitution, mainly at position 3 (see diagram in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme. Pyrroles are components of more complex macrocycles, including the porphyrinogens and products derived therefrom, including porphyrins of heme, the chlorins, bacteriochlorins, and chlorophylls. Properties Pyrrole is a colorless volatile liquid that darkens readily upon exposure to air, and is usually purified by distillation immediately before use. Pyrrole has a nutty odor. Pyrrole is a 5-membered aromatic heterocycle, like furan and thiophene. Unlike furan and thiophene, it has a dipole in which the positive end lies on the side of the heteroatom, with a dipole moment of 1.58 D. In CDCl3, it has ch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.png)