IR-spectrum on:
[Wikipedia]
[Google]
[Amazon]
 Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of
 Infrared spectroscopy exploits the fact that molecules absorb frequencies that are characteristic of their
Infrared spectroscopy exploits the fact that molecules absorb frequencies that are characteristic of their  In particular, in the Born–Oppenheimer and harmonic approximations, i.e. when the
In particular, in the Born–Oppenheimer and harmonic approximations, i.e. when the
 Simple
Simple

 It is typical to record spectrum of both the sample and a "reference". This step controls for a number of variables, e.g.
It is typical to record spectrum of both the sample and a "reference". This step controls for a number of variables, e.g.

/ref> There are other advantages, as well as some disadvantages, but virtually all modern infrared spectrometers are FTIR instruments.

 Nonlinear two-dimensional infrared spectroscopy is the infrared version of
Nonlinear two-dimensional infrared spectroscopy is the infrared version of
Infrared spectroscopy for organic chemistsOrganic compounds spectrum database
{{BranchesofSpectroscopy
 Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from around ...
radiation with matter
In classical physics and general chemistry, matter is any substance that has mass and takes up space by having volume. All everyday objects that can be touched are ultimately composed of atoms, which are made up of interacting subatomic partic ...
by absorption
Absorption may refer to:
Chemistry and biology
* Absorption (biology), digestion
**Absorption (small intestine)
*Absorption (chemistry), diffusion of particles of gas or liquid into liquid or solid materials
*Absorption (skin), a route by which ...
, emission
Emission may refer to:
Chemical products
* Emission of air pollutants, notably:
**Flue gas, gas exiting to the atmosphere via a flue
** Exhaust gas, flue gas generated by fuel combustion
** Emission of greenhouse gases, which absorb and emit radi ...
, or reflection Reflection or reflexion may refer to:
Science and technology
* Reflection (physics), a common wave phenomenon
** Specular reflection, reflection from a smooth surface
*** Mirror image, a reflection in a mirror or in water
** Signal reflection, in s ...
. It is used to study and identify chemical substance
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., wi ...
s or functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
s in solid, liquid, or gaseous forms. It can be used to characterize new materials or identify and verify known and unknown samples. The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer (or spectrophotometer) which produces an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance
Absorbance is defined as "the logarithm of the ratio of incident to transmitted radiant power through a sample (excluding the effects on cell walls)". Alternatively, for samples which scatter light, absorbance may be defined as "the negative lo ...
(or transmittance
Transmittance of the surface of a material is its effectiveness in transmitting radiant energy. It is the fraction of incident electromagnetic power that is transmitted through a sample, in contrast to the transmission coefficient, which is th ...
) on the vertical axis vs. frequency
Frequency is the number of occurrences of a repeating event per unit of time. It is also occasionally referred to as ''temporal frequency'' for clarity, and is distinct from ''angular frequency''. Frequency is measured in hertz (Hz) which is eq ...
, wavenumber
In the physical sciences, the wavenumber (also wave number or repetency) is the ''spatial frequency'' of a wave, measured in cycles per unit distance (ordinary wavenumber) or radians per unit distance (angular wavenumber). It is analogous to temp ...
or wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, tro ...
on the horizontal axis. Typical unit
Unit may refer to:
Arts and entertainment
* UNIT, a fictional military organization in the science fiction television series ''Doctor Who''
* Unit of action, a discrete piece of action (or beat) in a theatrical presentation
Music
* ''Unit'' (alb ...
s of wavenumber used in IR spectra are reciprocal centimeters Reciprocal length or inverse length is a quantity or measurement used in several branches of science and mathematics. As the reciprocal of length, common units used for this measurement include the reciprocal metre or inverse metre (symbol: m&min ...
, with the symbol cm−1. Units of IR wavelength are commonly given in micrometer Micrometer can mean:
* Micrometer (device), used for accurate measurements by means of a calibrated screw
* American spelling of micrometre
The micrometre ( international spelling as used by the International Bureau of Weights and Measures; ...
s (formerly called "microns"), symbol μm, which are related to the wavenumber in a reciprocal
Reciprocal may refer to:
In mathematics
* Multiplicative inverse, in mathematics, the number 1/''x'', which multiplied by ''x'' gives the product 1, also known as a ''reciprocal''
* Reciprocal polynomial, a polynomial obtained from another pol ...
way. A common laboratory instrument that uses this technique is a Fourier transform infrared (FTIR) spectrometer
A spectrometer () is a scientific instrument used to separate and measure spectral components of a physical phenomenon. Spectrometer is a broad term often used to describe instruments that measure a continuous variable of a phenomenon where the ...
. Two-dimensional IR is also possible as discussed below
Below may refer to:
*Earth
*Ground (disambiguation)
*Soil
*Floor
*Bottom (disambiguation)
Bottom may refer to:
Anatomy and sex
* Bottom (BDSM), the partner in a BDSM who takes the passive, receiving, or obedient role, to that of the top or ...
.
The infrared portion of the electromagnetic spectrum
The electromagnetic spectrum is the range of frequencies (the spectrum) of electromagnetic radiation and their respective wavelengths and photon energies.
The electromagnetic spectrum covers electromagnetic waves with frequencies ranging from ...
is usually divided into three regions; the near-, mid- and far- infrared, named for their relation to the visible spectrum. The higher-energy near-IR, approximately 14,000–4,000 cm−1 (0.7–2.5 μm wavelength) can excite overtone
An overtone is any resonant frequency above the fundamental frequency of a sound. (An overtone may or may not be a harmonic) In other words, overtones are all pitches higher than the lowest pitch within an individual sound; the fundamental i ...
or combination modes of molecular vibration
A molecular vibration is a periodic motion of the atoms of a molecule relative to each other, such that the center of mass of the molecule remains unchanged. The typical vibrational frequencies range from less than 1013 Hz to approximately 1014 Hz ...
s. The mid-infrared, approximately 4,000–400 cm−1 (2.5–25 μm) is generally used to study the fundamental vibrations and associated rotational–vibrational structure. The far-infrared, approximately 400–10 cm−1 (25–1,000 μm) has low energy and may be used for rotational spectroscopy
Rotational spectroscopy is concerned with the measurement of the energies of transitions between quantized rotational states of molecules in the gas phase. The spectra of polar molecules can be measured in absorption or emission by microwave sp ...
and low frequency vibrations. The region from 2–130 cm−1, bordering the microwave
Microwave is a form of electromagnetic radiation with wavelengths ranging from about one meter to one millimeter corresponding to frequencies between 300 MHz and 300 GHz respectively. Different sources define different frequency ran ...
region, is considered the terahertz region and may probe intermolecular vibrations. The names and classifications of these subregions are conventions, and are only loosely based on the relative molecular or electromagnetic properties.
Theory
structure
A structure is an arrangement and organization of interrelated elements in a material object or system, or the object or system so organized. Material structures include man-made objects such as buildings and machines and natural objects such as ...
. These absorptions occur at resonant frequencies
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillati ...
, i.e. the frequency of the absorbed radiation matches the vibrational frequency. The energies are affected by the shape of the molecular potential energy surface
A potential energy surface (PES) describes the energy of a system, especially a collection of atoms, in terms of certain parameters, normally the positions of the atoms. The surface might define the energy as a function of one or more coordinates; ...
s, the masses of the atoms, and the associated vibronic coupling
Vibronic coupling (also called nonadiabatic coupling or derivative coupling) in a molecule involves the interaction between electronic and nuclear vibrational motion. The term "vibronic" originates from the combination of the terms "vibrational" a ...
.
 In particular, in the Born–Oppenheimer and harmonic approximations, i.e. when the
In particular, in the Born–Oppenheimer and harmonic approximations, i.e. when the molecular Hamiltonian
In atomic, molecular, and optical physics and quantum chemistry, the molecular Hamiltonian is the Hamiltonian operator representing the energy of the electrons and nuclei in a molecule. This operator and the associated Schrödinger equation pl ...
corresponding to the electronic ground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. ...
can be approximated by a harmonic oscillator
In classical mechanics, a harmonic oscillator is a system that, when displaced from its Mechanical equilibrium, equilibrium position, experiences a restoring force ''F'' Proportionality (mathematics), proportional to the displacement ''x'':
\v ...
in the neighborhood of the equilibrium molecular geometry
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determ ...
, the resonant frequencies are associated with the normal modes
A normal mode of a dynamical system is a pattern of motion in which all parts of the system move sinusoidally with the same frequency and with a fixed phase relation. The free motion described by the normal modes takes place at fixed frequencies. ...
of vibration corresponding to the molecular electronic ground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. ...
potential energy surface.
The resonant frequencies are also related to the strength of the bond and the mass of the atoms at either end of it. Thus, the frequency of the vibrations are associated with a particular normal mode of motion and a particular bond type.
Number of vibrational modes
In order for a vibrational mode in a sample to be "IR active", it must be associated with changes in the dipole moment. A permanent dipole is not necessary, as the rule requires only a change in dipole moment. A molecule can vibrate in many ways, and each way is called a ''vibrational mode.'' For molecules with N number of atoms, geometrically linear molecules have 3''N'' – 5 degrees of vibrational modes, whereas nonlinear molecules have 3''N'' – 6 degrees of vibrational modes (also called vibrational degrees of freedom). As examples linearcarbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
(CO2) has 3 × 3 – 5 = 4, while non-linear water (H2O), has only 3 × 3 – 6 = 3. diatomic molecule
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear. Ot ...
s have only one bond and only one vibrational band. If the molecule is symmetrical, e.g. N2, the band is not observed in the IR spectrum, but only in the Raman spectrum
Raman spectroscopy () (named after Indian physicist C. V. Raman) is a Spectroscopy, spectroscopic technique typically used to determine vibrational modes of Molecule, molecules, although rotational and other low-frequency modes of systems may als ...
. Asymmetrical diatomic molecules, e.g. carbon monoxide ( CO), absorb in the IR spectrum. More complex molecules have many bonds, and their vibrational spectra are correspondingly more complex, i.e. big molecules have many peaks in their IR spectra.
The atoms in a CH2X2 group, commonly found in organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The ...
s and where X can represent any other atom, can vibrate in nine different ways. Six of these vibrations involve only the CH2 portion: two stretching modes (ν): symmetric (νs) and antisymmetric (νas); and four bending modes: scissoring (δ), rocking (ρ), wagging (ω) and twisting (τ), as shown below. Structures that do not have the two additional X groups attached have fewer modes because some modes are defined by specific relationships to those other attached groups. For example, in water, the rocking, wagging, and twisting modes do not exist because these types of motions of the H atoms represent simple rotation of the whole molecule rather than vibrations within it. In case of more complex molecules, out-of-plane (γ) vibrational modes can be also present.
These figures do not represent the "recoil
Recoil (often called knockback, kickback or simply kick) is the rearward thrust generated when a gun is being discharged. In technical terms, the recoil is a result of conservation of momentum, as according to Newton's third law the force requ ...
" of the C atoms, which, though necessarily present to balance the overall movements of the molecule, are much smaller than the movements of the lighter H atoms.
The simplest and most important or ''fundamental'' IR bands arise from the excitations of normal modes, the simplest distortions of the molecule, from the ground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. ...
with vibrational quantum number
A molecular vibration is a periodic motion of the atoms of a molecule relative to each other, such that the center of mass of the molecule remains unchanged. The typical vibrational frequencies range from less than 1013 Hz to approximately 1014 Hz ...
''v'' = 0 to the first excited state
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers to a ...
with vibrational quantum number ''v'' = 1. In some cases, overtone band
In vibrational spectroscopy, an overtone band is the spectral band that occurs in a vibrational spectrum of a molecule when the molecule makes a transition from the ground state (v=0) to the second excited state (v=2), where v is the vibrational q ...
s are observed. An overtone band arises from the absorption of a photon leading to a direct transition from the ground state to the second excited vibrational state (''v'' = 2). Such a band appears at approximately twice the energy of the fundamental band for the same normal mode. Some excitations, so-called ''combination modes'', involve simultaneous excitation of more than one normal mode. The phenomenon of Fermi resonance A Fermi resonance is the shifting of the energies and intensities of absorption bands in an infrared or Raman spectrum. It is a consequence of quantum mechanical wavefunction mixing. The phenomenon was explained by the Italian physicist Enrico Fermi ...
can arise when two modes are similar in energy; Fermi resonance results in an unexpected shift in energy and intensity of the bands etc.
Practical IR spectroscopy
The infrared spectrum of a sample is recorded by passing a beam of infrared light through the sample. When the frequency of the IR is the same as the vibrational frequency of a bond or collection of bonds, absorption occurs. Examination of the transmitted light reveals how much energy was absorbed at each frequency (or wavelength). This measurement can be achieved by scanning the wavelength range using amonochromator
A monochromator is an optical device that transmits a mechanically selectable narrow band of wavelengths of light or other radiation chosen from a wider range of wavelengths available at the input. The name is from the Greek roots ''mono-'', "si ...
. Alternatively, the entire wavelength range is measured using a Fourier transform
A Fourier transform (FT) is a mathematical transform that decomposes functions into frequency components, which are represented by the output of the transform as a function of frequency. Most commonly functions of time or space are transformed, ...
instrument and then a transmittance
Transmittance of the surface of a material is its effectiveness in transmitting radiant energy. It is the fraction of incident electromagnetic power that is transmitted through a sample, in contrast to the transmission coefficient, which is th ...
or absorbance
Absorbance is defined as "the logarithm of the ratio of incident to transmitted radiant power through a sample (excluding the effects on cell walls)". Alternatively, for samples which scatter light, absorbance may be defined as "the negative lo ...
spectrum is generated using a dedicated procedure.
This technique is commonly used for analyzing samples with covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
s. Simple spectra are obtained from samples with few IR active bonds and high levels of purity. More complex molecular structures lead to more absorption bands and more complex spectra.

Sample preparation
Gas samples
Gaseous samples require a sample cell with a long pathlength to compensate for the diluteness. The pathlength of the sample cell depends on the concentration of the compound of interest. A simple glass tube with length of 5 to 10 cm equipped with infrared-transparent windows at both ends of the tube can be used for concentrations down to several hundred ppm. Sample gas concentrations well below ppm can be measured with a White's cell in which the infrared light is guided with mirrors to travel through the gas. White's cells are available with optical pathlength starting from 0.5 m up to hundred meters.Liquid samples
Liquid samples can be sandwiched between two plates of a salt (commonlysodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
, or common salt, although a number of other salts such as potassium bromide
Potassium bromide ( K Br) is a salt, widely used as an anticonvulsant and a sedative in the late 19th and early 20th centuries, with over-the-counter use extending to 1975 in the US. Its action is due to the bromide ion (sodium bromide is equall ...
or calcium fluoride
Calcium fluoride is the inorganic compound of the elements calcium and fluorine with the formula CaF2. It is a white insoluble solid. It occurs as the mineral fluorite (also called fluorspar), which is often deeply coloured owing to impurities. ...
are also used).
The plates are transparent to the infrared light and do not introduce any lines onto the spectra.
Solid samples
Solid samples can be prepared in a variety of ways. One common method is to crush the sample with an oily mulling agent (usually mineral oilNujol
Nujol is a brand of mineral oil by Plough Inc., cas number 8012-95-1, and density 0.838 g/mL at 25 °C, used in infrared spectroscopy. It is a heavy paraffin oil so it is chemically inert and has a relatively uncomplicated IR spectrum, with ma ...
). A thin film of the mull is applied onto salt plates and measured. The second method is to grind a quantity of the sample with a specially purified salt (usually potassium bromide
Potassium bromide ( K Br) is a salt, widely used as an anticonvulsant and a sedative in the late 19th and early 20th centuries, with over-the-counter use extending to 1975 in the US. Its action is due to the bromide ion (sodium bromide is equall ...
) finely (to remove scattering effects from large crystals). This powder mixture is then pressed in a mechanical press
Press may refer to:
Media
* Print media or news media, commonly called "the press"
* Printing press, commonly called "the press"
* Press (newspaper), a list of newspapers
* Press TV, an Iranian television network
People
* Press (surname), a famil ...
to form a translucent pellet through which the beam of the spectrometer can pass. A third technique is the "cast film" technique, which is used mainly for polymeric materials. The sample is first dissolved in a suitable, non-hygroscopic
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance ...
solvent. A drop of this solution is deposited on surface of KBr KBR can stand for:
* KBR (company), formerly Kellogg, Brown & Root, US
* KBR (news agency), an Indonesian radio news agency
* KBR Park, Hyderabad, India
* Kafa language, spoken in Ethiopia
* Key-based routing in computer networking
* Potassium brom ...
or NaCl
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
cell. The solution is then evaporated to dryness and the film formed on the cell is analysed directly. Care is important to ensure that the film is not too thick otherwise light cannot pass through. This technique is suitable for qualitative analysis. The final method is to use microtomy
A microtome (from the Greek ''mikros'', meaning "small", and ''temnein'', meaning "to cut") is a cutting tool used to produce extremely thin slices of material known as ''sections''. Important in science, microtomes are used in microscopy, allo ...
to cut a thin (20–100 μm) film from a solid sample. This is one of the most important ways of analysing failed plastic products for example because the integrity of the solid is preserved.
In photoacoustic spectroscopy
Photoacoustic spectroscopy is the measurement of the effect of absorbed electromagnetic energy (particularly of light) on matter by means of acoustic detection. The discovery of the photoacoustic effect dates to 1880 when Alexander Graham Bell sho ...
the need for sample treatment is minimal. The sample, liquid or solid, is placed into the sample cup which is inserted into the photoacoustic cell which is then sealed for the measurement. The sample may be one solid piece, powder or basically in any form for the measurement. For example, a piece of rock can be inserted into the sample cup and the spectrum measured from it.
Comparing to a reference
infrared detector
An infrared detector is a detector that reacts to infrared (IR) radiation. The two main types of detectors are thermal and photonic (photodetectors).
The thermal effects of the incident IR radiation can be followed through many temperature depen ...
, which may affect the spectrum. The reference measurement makes it possible to eliminate the instrument influence.
The appropriate "reference" depends on the measurement and its goal. The simplest reference measurement is to simply remove the sample (replacing it by air). However, sometimes a different reference is more useful. For example, if the sample is a dilute solute dissolved in water in a beaker, then a good reference measurement might be to measure pure water in the same beaker. Then the reference measurement would cancel out not only all the instrumental properties (like what light source is used), but also the light-absorbing and light-reflecting properties of the water and beaker, and the final result would just show the properties of the solute (at least approximately).
A common way to compare to a reference is sequentially: first measure the reference, then replace the reference by the sample and measure the sample. This technique is not perfectly reliable; if the infrared lamp is a bit brighter during the reference measurement, then a bit dimmer during the sample measurement, the measurement will be distorted. More elaborate methods, such as a "two-beam" setup (see figure), can correct for these types of effects to give very accurate results. The Standard addition
The method of standard addition is a type of quantitative analysis approach often used in analytical chemistry whereby the standard is added directly to the aliquots of analyzed sample. This method is used in situations where sample matrix also ...
method can be used to statistically cancel these errors.
Nevertheless, among different absorption based techniques which are used for gaseous species detection, Cavity ring-down spectroscopy Cavity ring-down spectroscopy (CRDS) is a highly sensitive optical spectroscopic technique that enables measurement of absolute optical extinction by samples that scatter and absorb light. It has been widely used to study gaseous samples which a ...
(CRDS) can be used as a calibration free method. The fact that CRDS is based on the measurements of photon life-times (and not the laser intensity) makes it needless for any calibration and comparison with a reference
FTIR
Fourier transform
A Fourier transform (FT) is a mathematical transform that decomposes functions into frequency components, which are represented by the output of the transform as a function of frequency. Most commonly functions of time or space are transformed, ...
infrared (FTIR) spectroscopy is a measurement technique that allows one to record infrared spectra. Infrared light is guided through an interferometer
Interferometry is a technique which uses the ''interference'' of superimposed waves to extract information. Interferometry typically uses electromagnetic waves and is an important investigative technique in the fields of astronomy, fiber op ...
and then through the sample (or vice versa). A moving mirror inside the apparatus alters the distribution of infrared light that passes through the interferometer. The signal directly recorded, called an "interferogram", represents light output as a function of mirror position. A data-processing technique called Fourier transform
A Fourier transform (FT) is a mathematical transform that decomposes functions into frequency components, which are represented by the output of the transform as a function of frequency. Most commonly functions of time or space are transformed, ...
turns this raw data into the desired result (the sample's spectrum): Light output as a function of infrared wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, tro ...
(or equivalently, wavenumber
In the physical sciences, the wavenumber (also wave number or repetency) is the ''spatial frequency'' of a wave, measured in cycles per unit distance (ordinary wavenumber) or radians per unit distance (angular wavenumber). It is analogous to temp ...
). As described above, the sample's spectrum is always compared to a reference.
An alternate method for acquiring spectra is the "dispersive" or "scanning monochromator
A monochromator is an optical device that transmits a mechanically selectable narrow band of wavelengths of light or other radiation chosen from a wider range of wavelengths available at the input. The name is from the Greek roots ''mono-'', "si ...
" method. In this approach, the sample is irradiated sequentially with various single wavelengths. The dispersive method is more common in UV-Vis spectroscopy, but is less practical in the infrared than the FTIR method. One reason that FTIR is favored is called "Fellgett's advantage Fellgett's advantage or the multiplex advantage is an improvement in signal-to-noise ratio (SNR) that is gained when taking multiplexed measurements rather than direct measurements. The name is derived from P. B. Fellgett, who first made the observ ...
" or the "multiplex advantage": The information at all frequencies is collected simultaneously, improving both speed and signal-to-noise ratio
Signal-to-noise ratio (SNR or S/N) is a measure used in science and engineering that compares the level of a desired signal to the level of background noise. SNR is defined as the ratio of signal power to the noise power, often expressed in deci ...
. Another is called "Jacquinot's Throughput Advantage": A dispersive measurement requires detecting much lower light levels than an FTIR measurement.''Chromatography/Fourier transform infrared spectroscopy and its applications'', by Robert White, p7/ref> There are other advantages, as well as some disadvantages, but virtually all modern infrared spectrometers are FTIR instruments.
Infrared microscopy
Various forms ofinfrared microscopy
Microscopy is the technical field of using microscopes to view objects and areas of objects that cannot be seen with the naked eye (objects that are not within the resolution range of the normal eye). There are three well-known branches of micr ...
exist. These include IR versions of sub-diffraction microscopyH M Pollock and S G Kazarian, Microspectroscopy in the Mid-Infrared, in Encyclopedia of Analytical Chemistry (Robert A. Meyers, Ed, 1-26 (2014), John Wiley & Sons Ltd, such as IR NSOM, photothermal microspectroscopy Photothermal microspectroscopy (PTMS), alternatively known as photothermal temperature fluctuation (PTTF), is derived from two parent instrumental techniques: infrared spectroscopy and atomic force microscopy (AFM). In one particular type of AFM, kn ...
, Nano-FTIR
Nano-FTIR (nanoscale Fourier transform infrared spectroscopy) is a scanning probe technique that utilizes as a combination of two techniques: Fourier transform infrared spectroscopy (FTIR) and scattering-type scanning near-field optical microsco ...
and atomic force microscope based infrared spectroscopy (AFM-IR).
Other methods in molecular vibrational spectroscopy
Infrared spectroscopy is not the only method of studying molecular vibrational spectra.Raman spectroscopy
Raman spectroscopy () (named after Indian physicist C. V. Raman) is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Raman sp ...
involves an inelastic scattering In chemistry, nuclear physics, and particle physics, inelastic scattering is a fundamental scattering process in which the kinetic energy of an incident particle is not conserved (in contrast to elastic scattering). In an inelastic scattering proces ...
process in which only part of the energy of an incident photon is absorbed by the molecule, and the remaining part is scattered and detected. The energy difference corresponds to absorbed vibrational energy.
The selection rule
In physics and chemistry, a selection rule, or transition rule, formally constrains the possible transitions of a system from one quantum state to another. Selection rules have been derived for electromagnetic transitions in molecules, in atoms, in ...
s for infrared and for Raman spectroscopy are different at least for some molecular symmetries, so that the two methods are complementary in that they observe vibrations of different symmetries.
Another method is electron energy loss spectroscopy
In electron energy loss spectroscopy (EELS) a material is exposed to a beam of electrons with a known, narrow range of kinetic energies. Some of the electrons will undergo inelastic scattering, which means that they lose energy and have their pa ...
(EELS), in which the energy absorbed is provided by an inelastically scattered electron rather than a photon. This method is useful for studying vibrations of molecules adsorbed
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a ...
on a solid surface.
Recently, high-resolution EELS (HREELS) has emerged as a technique for performing vibrational spectroscopy in a transmission electron microscope
Transmission electron microscopy (TEM) is a microscopy technique in which a beam of electrons is transmitted through a specimen to form an image. The specimen is most often an ultrathin section less than 100 nm thick or a suspension on a gr ...
(TEM). In combination with the high spatial resolution of the TEM, unprecedented experiments have been performed, such as nano-scale temperature measurements, mapping of isotopically labeled molecules, mapping of phonon modes in position- and momentum-space, vibrational surface and bulk mode mapping on nanocubes, and investigations of polariton
In physics, polaritons are quasiparticles resulting from strong coupling of electromagnetic waves with an electric or magnetic dipole-carrying excitation. They are an expression of the common quantum phenomenon known as level repulsion, also k ...
modes in van der Waals crystals.
Analysis of vibrational modes that are IR-inactive but appear in inelastic neutron scattering
Neutron scattering, the irregular dispersal of free neutrons by matter, can refer to either the naturally occurring physical process itself or to the man-made experimental techniques that use the natural process for investigating materials. Th ...
is also possible at high spatial resolution using EELS. Although the spatial resolution of HREELs is very high, the bands are extremely broad compared to other techniques.
Computational infrared microscopy
By using computersimulation
A simulation is the imitation of the operation of a real-world process or system over time. Simulations require the use of Conceptual model, models; the model represents the key characteristics or behaviors of the selected system or proc ...
s and normal mode
A normal mode of a dynamical system is a pattern of motion in which all parts of the system move sinusoidally with the same frequency and with a fixed phase relation. The free motion described by the normal modes takes place at fixed frequencies. ...
analysis it is possible to calculate theoretical frequencies of molecules.
Absorption bands
IR spectroscopy is often used to identify structures becausefunctional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
s give rise to characteristic bands both in terms of intensity and position (frequency). The positions of these bands are summarized in correlation tables as shown below.
Regions
A spectrograph is often interpreted as having two regions. *functional group region In the functional region there are one to a few troughs per functional group. *fingerprint region In the fingerprint region there are many troughs which form an intricate pattern which can be used like a fingerprint to determine the compound.Badger's rule
For many kinds of samples, the assignments are known, i.e. which bond deformation(s) are associated with which frequency. In such cases further information can be gleaned about the strength on a bond, relying on the empirical guideline called Badger's Rule. Originally published byRichard McLean Badger Richard McLean Badger (4 May 1896 – 26 November 1974) was an American professor at Caltech and a chemist who specialized in molecular spectroscopy with X rays and infrared radiation. He made pioneering studies on the modes of vibrations and spectr ...
in 1934, this rule states that the strength of a bond (in terms of force constant) correlates with the bond length. That is, increase in bond strength leads to corresponding bond shortening and vice versa.
Uses and applications
Infrared spectroscopy is a simple and reliable technique widely used in both organic and inorganic chemistry, in research and industry. In catalysis research it is a very useful tool to characterize the catalyst, as well as to detect intermediates and products during the catalytic reaction. It is used in quality control, dynamic measurement, and monitoring applications such as the long-term unattended measurement of CO2 concentrations in greenhouses and growth chambers by infrared gas analyzers. It is also used inforensic analysis
Forensic science, also known as criminalistics, is the application of science to criminal and civil laws, mainly—on the criminal side—during criminal investigation, as governed by the legal standards of admissible evidence and criminal p ...
in both criminal and civil cases, for example in identifying polymer degradation
Polymer degradation is the reduction in the physical properties of a polymer, such as strength, caused by changes in its chemical composition. Polymers and particularly plastics are subject to degradation at all stages of their product life cycle ...
. It can be used in determining the blood alcohol content
Blood alcohol content (BAC), also called blood alcohol concentration or blood alcohol level, is a measurement of alcohol intoxication used for legal or medical purposes; it is expressed as mass of alcohol per volume or mass of blood. For exampl ...
of a suspected drunk driver.
IR-spectroscopy has been successfully used in analysis and identification of pigments
A pigment is a colored material that is completely or nearly insoluble in water. In contrast, dyes are typically soluble, at least at some stage in their use. Generally dyes are often organic compounds whereas pigments are often inorganic compou ...
in paintings
Painting is the practice of applying paint, pigment, color or other medium to a solid surface (called the "matrix" or "support"). The medium is commonly applied to the base with a brush, but other implements, such as knives, sponges, and ai ...
and other art objects such as illuminated manuscripts
An illuminated manuscript is a formally prepared document where the text is often supplemented with flourishes such as borders and miniature illustrations. Often used in the Roman Catholic Church for prayers, liturgical services and psalms, the ...
.
A useful way of analyzing solid samples without the need for cutting samples uses ATR or attenuated total reflectance
Attenuated total reflection (ATR) is a sampling technique used in conjunction with infrared spectroscopy which enables samples to be examined directly in the solid or liquid state without further preparation.
ATR uses a property of total inter ...
spectroscopy. Using this approach, samples are pressed against the face of a single crystal. The infrared radiation passes through the crystal and only interacts with the sample at the interface between the two materials.
With increasing technology in computer filtering and manipulation of the results, samples in solution can now be measured accurately (water produces a broad absorbance across the range of interest, and thus renders the spectra unreadable without this computer treatment).
Some instruments also automatically identify the substance being measured from a store of thousands of reference spectra held in storage.
Infrared spectroscopy is also useful in measuring the degree of polymerization in polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
manufacture. Changes in the character or quantity of a particular bond are assessed by measuring at a specific frequency over time. Modern research instruments can take infrared measurements across the range of interest as frequently as 32 times a second. This can be done whilst simultaneous measurements are made using other techniques. This makes the observations of chemical reactions and processes quicker and more accurate.
Infrared spectroscopy has also been successfully utilized in the field of semiconductor microelectronics: for example, infrared spectroscopy can be applied to semiconductors like silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic tab ...
, gallium arsenide
Gallium arsenide (GaAs) is a III-V direct band gap semiconductor with a Zincblende (crystal structure), zinc blende crystal structure.
Gallium arsenide is used in the manufacture of devices such as microwave frequency integrated circuits, monoli ...
, gallium nitride
Gallium nitride () is a binary III/ V direct bandgap semiconductor commonly used in blue light-emitting diodes since the 1990s. The compound is a very hard material that has a Wurtzite crystal structure. Its wide band gap of 3.4 eV affords it ...
, zinc selenide
Zinc selenide (ZnSe) is a light-yellow, solid compound comprising zinc (Zn) and selenium (Se). It is an intrinsic semiconductor with a band gap of about 2.70 eV at . ZnSe rarely occurs in nature, and is found in the mineral that was named af ...
, amorphous silicon, silicon nitride
Silicon nitride is a chemical compound of the elements silicon and nitrogen. is the most thermodynamically stable and commercially important of the silicon nitrides, and the term "silicon nitride" commonly refers to this specific composition. It ...
, etc.
Another important application of Infrared Spectroscopy is in the food industry
The food industry is a complex, global network of diverse businesses that supplies most of the food consumed by the world's population. The food industry today has become highly diversified, with manufacturing ranging from small, traditiona ...
to measure the concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', ''molar concentration'', ''number concentration'', an ...
of various compounds in different food products.
The instruments are now small, and can be transported, even for use in field trials.
Infrared Spectroscopy is also used in gas leak detection devices such as the DP-IR and EyeCGAs. These devices detect hydrocarbon gas leaks in the transportation of natural gas and crude oil.
In February 2014, NASA
The National Aeronautics and Space Administration (NASA ) is an independent agency of the US federal government responsible for the civil space program, aeronautics research, and space research.
NASA was established in 1958, succeeding t ...
announced a greatly upgraded database, based on IR spectroscopy, for tracking polycyclic aromatic hydrocarbons
A polycyclic aromatic hydrocarbon (PAH) is a class of organic compounds that is composed of multiple aromatic rings. The simplest representative is naphthalene, having two aromatic rings and the three-ring compounds anthracene and phenanthrene. P ...
(PAHs) in the universe
The universe is all of space and time and their contents, including planets, stars, galaxies, and all other forms of matter and energy. The Big Bang theory is the prevailing cosmological description of the development of the universe. Acc ...
. According to scientists, more than 20% of the carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
in the universe may be associated with PAHs, possible starting materials for the formation
Formation may refer to:
Linguistics
* Back-formation, the process of creating a new lexeme by removing or affixes
* Word formation, the creation of a new word by adding affixes
Mathematics and science
* Cave formation or speleothem, a secondar ...
of life
Life is a quality that distinguishes matter that has biological processes, such as signaling and self-sustaining processes, from that which does not, and is defined by the capacity for growth, reaction to stimuli, metabolism, energ ...
. PAHs seem to have been formed shortly after the Big Bang
The Big Bang event is a physical theory that describes how the universe expanded from an initial state of high density and temperature. Various cosmological models of the Big Bang explain the evolution of the observable universe from the ...
, are widespread throughout the universe, and are associated with new stars and exoplanets
An exoplanet or extrasolar planet is a planet outside the Solar System. The first possible evidence of an exoplanet was noted in 1917 but was not recognized as such. The first confirmation of detection occurred in 1992. A different planet, init ...
.
Infrared spectroscopy is an important analysis method in the recycling process of household waste plastics
Plastic pollution is the accumulation of plastic objects and particles (e.g. plastic bottles, bags and microbeads) in the Earth's environment that adversely affects humans, wildlife and their habitat. Plastics that act as pollutants are catego ...
, and a convenient stand-off method to sort plastic of different polymers (PET
A pet, or companion animal, is an animal kept primarily for a person's company or entertainment rather than as a working animal, livestock, or a laboratory animal. Popular pets are often considered to have attractive appearances, intelligence, ...
, HDPE
High-density polyethylene (HDPE) or polyethylene high-density (PEHD) is a thermoplastic polymer produced from the monomer ethylene. It is sometimes called "alkathene" or "polythene" when used for HDPE pipes. With a high strength-to-density ratio, ...
, ...).
Other developments include a miniature IR-spectrometer that's linked to a cloud based database and suitable for personal everyday use, and NIR-spectroscopic chips that can be embedded in smartphones and various gadgets.
Isotope effects
The different isotopes in a particular species may exhibit different fine details in infrared spectroscopy. For example, the O–O stretching frequency (in reciprocal centimeters) of oxyhemocyanin
Hemocyanins (also spelled haemocyanins and abbreviated Hc) are proteins that transport oxygen throughout the bodies of some invertebrate animals. These metalloproteins contain two copper atoms that reversibly bind a single oxygen molecule (O2) ...
is experimentally determined to be 832 and 788 cm−1 for ν(16O–16O) and ν(18O–18O), respectively.
By considering the O–O bond as a spring, the frequency of absorbance can be calculated as a wavenumber
In the physical sciences, the wavenumber (also wave number or repetency) is the ''spatial frequency'' of a wave, measured in cycles per unit distance (ordinary wavenumber) or radians per unit distance (angular wavenumber). It is analogous to temp ...
frequency/(speed of light)
:
where ''k'' is the spring constant for the bond, ''c'' is the speed of light, and ''μ'' is the reduced mass
In physics, the reduced mass is the "effective" Mass#Inertial mass, inertial mass appearing in the two-body problem of Newtonian mechanics. It is a quantity which allows the two-body problem to be solved as if it were a one-body problem. Note, how ...
of the A–B system:
:
( is the mass of atom ).
The reduced masses for 16O–16O and 18O–18O can be approximated as 8 and 9 respectively. Thus
:
The effect of isotopes, both on the vibration and the decay dynamics, has been found to be stronger than previously thought. In some systems, such as silicon and germanium, the decay of the anti-symmetric stretch mode of interstitial oxygen involves the symmetric stretch mode with a strong isotope dependence. For example, it was shown that for a natural silicon sample, the lifetime of the anti-symmetric vibration is 11.4 ps. When the isotope of one of the silicon atoms is increased to 29Si, the lifetime increases to 19 ps. In similar manner, when the silicon atom is changed to 30Si, the lifetime becomes 27 ps.
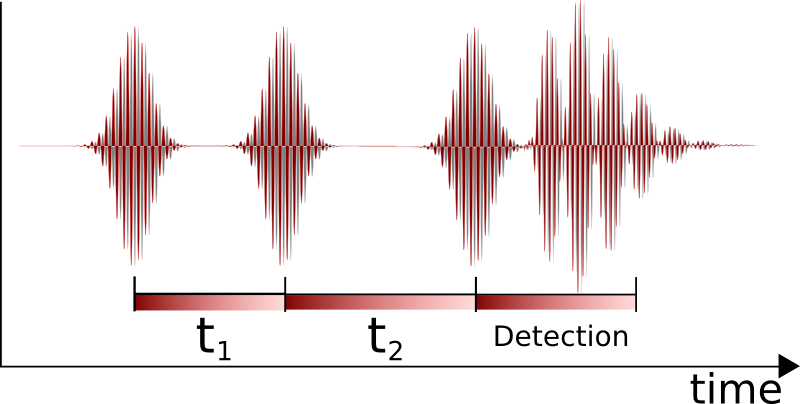
Two-dimensional IR
Two-dimensional infrared correlation spectroscopy analysis combines multiple samples of infrared spectra to reveal more complex properties. By extending the spectral information of a perturbed sample, spectral analysis is simplified and resolution is enhanced. The 2D synchronous and 2D asynchronous spectra represent a graphical overview of the spectral changes due to a perturbation (such as a changing concentration or changing temperature) as well as the relationship between the spectral changes at two different wavenumbers. Nonlinear two-dimensional infrared spectroscopy is the infrared version of
Nonlinear two-dimensional infrared spectroscopy is the infrared version of correlation spectroscopy Two-dimensional nuclear magnetic resonance spectroscopy (2D NMR) is a set of nuclear magnetic resonance spectroscopy (NMR) methods which give data plotted in a space defined by two frequency axes rather than one. Types of 2D NMR include correlation ...
. Nonlinear two-dimensional infrared spectroscopy is a technique that has become available with the development of femtosecond
A femtosecond is a unit of time in the International System of Units (SI) equal to 10 or of a second; that is, one quadrillionth, or one millionth of one billionth, of a second. For context, a femtosecond is to a second as a second is to about 31. ...
infrared laser pulses. In this experiment, first a set of pump pulses is applied to the sample. This is followed by a waiting time during which the system is allowed to relax. The typical waiting time lasts from zero to several picoseconds, and the duration can be controlled with a resolution of tens of femtoseconds. A probe pulse is then applied, resulting in the emission of a signal from the sample. The nonlinear two-dimensional infrared spectrum is a two-dimensional correlation plot of the frequency ω1 that was excited by the initial pump pulses and the frequency ω3 excited by the probe pulse after the waiting time. This allows the observation of coupling between different vibrational modes; because of its extremely fine time resolution, it can be used to monitor molecular dynamics on a picosecond timescale. It is still a largely unexplored technique and is becoming increasingly popular for fundamental research.
As with two-dimensional nuclear magnetic resonance ( 2DNMR) spectroscopy, this technique spreads the spectrum in two dimensions and allows for the observation of cross peaks that contain information on the coupling between different modes. In contrast to 2DNMR, nonlinear two-dimensional infrared spectroscopy also involves the excitation to overtones. These excitations result in excited state absorption peaks located below the diagonal and cross peaks. In 2DNMR, two distinct techniques, COSY
Cosy may refer to
* Tea cosy, a cover for a teapot
* Cozy mystery, a subgenre of crime fiction
* Cosy catastrophe, post-apocalyptic science fiction style
* Correlation spectroscopy (COSY)
* CoSy (Conferencing System), an early computer conferenc ...
and NOESY Two-dimensional nuclear magnetic resonance spectroscopy (2D NMR) is a set of nuclear magnetic resonance spectroscopy (NMR) methods which give data plotted in a space defined by two frequency axes rather than one. Types of 2D NMR include correlation ...
, are frequently used. The cross peaks in the first are related to the scalar coupling, while in the latter they are related to the spin transfer between different nuclei. In nonlinear two-dimensional infrared spectroscopy, analogs have been drawn to these 2DNMR techniques. Nonlinear two-dimensional infrared spectroscopy with zero waiting time corresponds to COSY, and nonlinear two-dimensional infrared spectroscopy with finite waiting time allowing vibrational population transfer corresponds to NOESY. The COSY variant of nonlinear two-dimensional infrared spectroscopy has been used for determination of the secondary structure content of proteins.
See also
*Applied spectroscopy
Applied spectroscopy is the application of various spectroscopy, spectroscopic methods for the detection and identification of different chemical element, elements or Chemical compound, compounds to solve problems in fields like forensics, medic ...
*Astrochemistry
Astrochemistry is the study of the abundance and reactions of molecules in the Universe, and their interaction with radiation. The discipline is an overlap of astronomy and chemistry. The word "astrochemistry" may be applied to both the Solar Syst ...
*Atomic and molecular astrophysics
Atomic astrophysics is concerned with performing atomic physics calculations that will be useful to astronomers and using atomic data to interpret astronomical observations. Atomic physics plays a key role in astrophysics as astronomers' only info ...
* Atomic force microscopy based infrared spectroscopy (AFM-IR)
*Cosmochemistry
Cosmochemistry (from Greek κόσμος ''kósmos'', "universe" and χημεία ''khemeía'') or chemical cosmology is the study of the chemical composition of matter in the universe and the processes that led to those compositions. This is done ...
*Far-infrared astronomy
Far-infrared astronomy is the branch of astronomy and astrophysics that deals with objects visible in far-infrared radiation (extending from 30 μm towards submillimeter wavelengths around 450 μm).
In the far-infrared, stars are not especi ...
*Forensic chemistry
Forensic chemistry is the application of chemistry and its subfield, forensic toxicology, in a legal setting. A forensic chemist can assist in the identification of unknown materials found at a crime scene. Specialists in this field have a wide ...
*Forensic engineering
Forensic engineering has been defined as ''"the investigation of failures - ranging from serviceability to catastrophic - which may lead to legal activity, including both civil and criminal".'' It includes the investigation of materials, product ...
*Forensic polymer engineering
Forensic polymer engineering is the study of failure in polymeric products. The topic includes the fracture of plastic products, or any other reason why such a product fails in service, or fails to meet its specification. The subject focuses on t ...
*Infrared astronomy
Infrared astronomy is a sub-discipline of astronomy which specializes in the observation and analysis of astronomical objects using infrared (IR) radiation. The wavelength of infrared light ranges from 0.75 to 300 micrometers, and falls in betwee ...
*Infrared microscopy
Microscopy is the technical field of using microscopes to view objects and areas of objects that cannot be seen with the naked eye (objects that are not within the resolution range of the normal eye). There are three well-known branches of micr ...
*Infrared multiphoton dissociation
Infrared multiple photon dissociation (IRMPD) is a technique used in mass spectrometry to fragment molecules in the gas phase usually for structural analysis of the original (parent) molecule.
How it works
An infrared laser is directed through ...
*Infrared photodissociation spectroscopy Infrared photodissociation (IRPD) spectroscopy uses infrared radiation to break bonds in ions, ( photodissociate), within a mass spectrometer. IRPD spectroscopy has been shown to use electron ionization, corona discharge, and electrospray ionization ...
* Infrared spectroscopy correlation table
*Infrared spectroscopy of metal carbonyls
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe c ...
*Near-infrared spectroscopy
Near-infrared spectroscopy (NIRS) is a spectroscopic method that uses the near-infrared region of the electromagnetic spectrum (from 780 nm to 2500 nm). Typical applications include medical and physiological diagnostics and research incl ...
*Nuclear resonance vibrational spectroscopy
Nuclear resonance vibrational spectroscopy is a synchrotron-based technique that probes vibrational energy levels. The technique, often called NRVS, is specific for samples that contain nuclei that respond to Mössbauer spectroscopy, most commonl ...
*Photothermal microspectroscopy Photothermal microspectroscopy (PTMS), alternatively known as photothermal temperature fluctuation (PTTF), is derived from two parent instrumental techniques: infrared spectroscopy and atomic force microscopy (AFM). In one particular type of AFM, kn ...
*Raman spectroscopy
Raman spectroscopy () (named after Indian physicist C. V. Raman) is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Raman sp ...
* Rotational-vibrational spectroscopy
*Time-resolved spectroscopy In physics and physical chemistry, time-resolved spectroscopy is the study of dynamic processes in materials or chemical compounds by means of spectroscopic techniques. Most often, processes are studied after the illumination of a material occurs, ...
*Vibrational spectroscopy of linear molecules To determine the vibrational spectroscopy of linear molecules, the rotation and vibration of linear molecules are taken into account to predict which vibrational (normal) modes are active in the infrared spectrum and the Raman spectrum.
Degrees of ...
References
External links
Infrared spectroscopy for organic chemists
{{BranchesofSpectroscopy