|
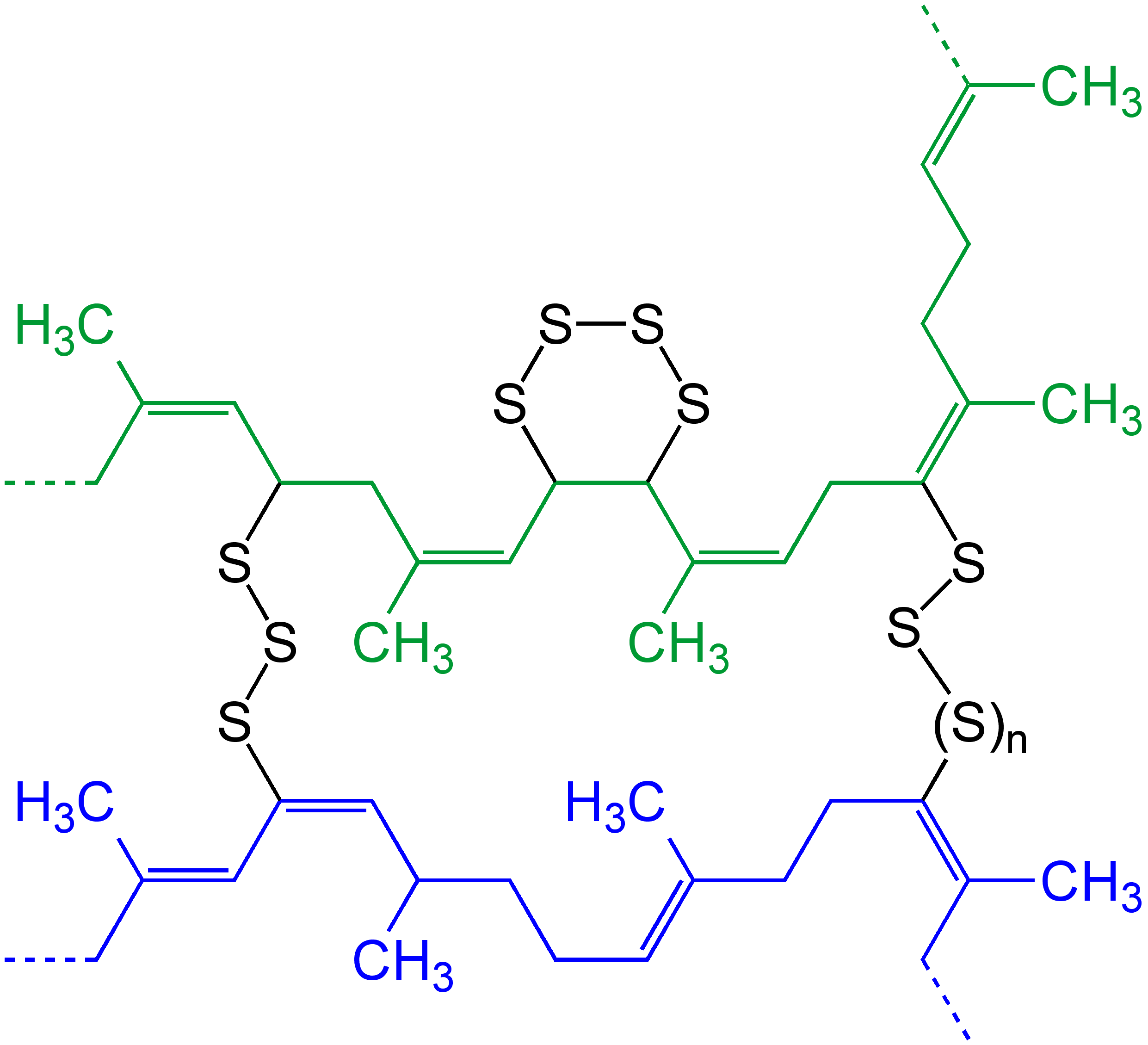
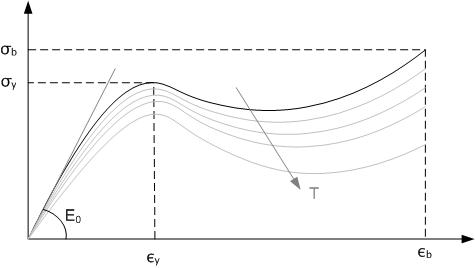
Polymer Degradation
Polymer degradation is the reduction in the physical properties of a polymer, such as strength, caused by changes in its chemical composition. Polymers and particularly plastics are subject to degradation at all stages of their product life cycle, including during their initial processing, use, disposal into the environment and recycling. The rate of this degradation varies significantly; biodegradation can take decades, whereas some industrial processes can completely decompose a polymer in hours. Technologies have been developed to both inhibit or promote degradation. For instance, polymer stabilizers ensure plastic items are produced with the desired properties, extend their useful lifespans, and facilitate their recycling. Conversely, biodegradable additives accelerate the degradation of plastic waste by improving its biodegradability. Some forms of plastic recycling can involve the complete degradation of a polymer back into monomers or other chemicals. In general, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer (; Greek ''poly-'', "many" + '' -mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. The term "polymer" derives from the Greek word πολύς (''polus'', meaning "many, much") and μέρος (''meros'', mean ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plastics Market Share En
Plastics are a wide range of synthetic or semi-synthetic materials that use polymers as a main ingredient. Their plasticity makes it possible for plastics to be moulded, extruded or pressed into solid objects of various shapes. This adaptability, plus a wide range of other properties, such as being lightweight, durable, flexible, and inexpensive to produce, has led to its widespread use. Plastics typically are made through human industrial systems. Most modern plastics are derived from fossil fuel-based chemicals like natural gas or petroleum; however, recent industrial methods use variants made from renewable materials, such as corn or cotton derivatives. 9.2 billion tonnes of plastic are estimated to have been made between 1950 and 2017. More than half this plastic has been produced since 2004. In 2020, 400 million tonnes of plastic were produced. If global trends on plastic demand continue, it is estimated that by 2050 annual global plastic production will reach over 1,100 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Condensation Polymer
In polymer chemistry, condensation polymers are any kind of polymers whose process of polymerization involves a condensation reaction (i.e. a small molecule, such as water or methanol, is produced as a byproduct). Condensation polymers are formed by polycondensation, when the polymer is formed by condensation reactions between species of all degrees of polymerization, or by condensative chain polymerization, when the polymer is formed by sequential addition of monomers to an active site in a chain reaction. The main alternative forms of polymerization are chain polymerization and polyaddition, both of which give addition polymers. Condensation polymerization is a form of step-growth polymerization. Linear polymers are produced from bifunctional monomers, i.e. compounds with two reactive end-groups. Common condensation polymers include polyamides, polyacetals, and proteins. Polyamides One important class of condensation polymers are polyamides. They arise from the reac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Addition Polymer
In polymer chemistry, an addition polymer is a polymer that forms by simple linking of monomers ''without'' the co-generation of other products. Addition polymerization differs from condensation polymerization, which ''does'' co-generate a product, usually water. Addition polymers can be formed by chain polymerization, when the polymer is formed by the sequential addition of monomer units to an active site in a chain reaction, or by polyaddition, when the polymer is formed by addition reactions between species of all degrees of polymerization. Addition polymers are formed by the addition of some simple monomer units repeatedly. Generally polymers are unsaturated compounds like alkenes, alkalines etc. The addition polymerization mainly takes place in free radical mechanism. The free radical mechanism of addition polymerization completed by three steps i.e. Initiation of free radical, Chain propagation, Termination of chain. Polyolefins Many common addition polymers are formed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cross-linked
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural polymers (such as proteins). In polymer chemistry "cross-linking" usually refers to the use of cross-links to promote a change in the polymers' physical properties. When "crosslinking" is used in the biological field, it refers to the use of a probe to link proteins together to check for protein–protein interactions, as well as other creative cross-linking methodologies. Although the term is used to refer to the "linking of polymer chains" for both sciences, the extent of crosslinking and specificities of the crosslinking agents vary greatly. As with all science, there are overlaps, and the following delineations are a starting point to understanding the subtleties. Polymer chemistry Crosslinking is the general term for the process o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermoset
In materials science, a thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening ("curing") a soft solid or viscous liquid prepolymer (resin). Curing is induced by heat or suitable radiation and may be promoted by high pressure, or mixing with a catalyst. Heat is not necessarily applied externally, but is often generated by the reaction of the resin with a curing agent (''catalyst'', '' hardener''). Curing results in chemical reactions that create extensive cross-linking between polymer chains to produce an infusible and insoluble polymer network. The starting material for making thermosets is usually malleable or liquid prior to curing, and is often designed to be molded into the final shape. It may also be used as an adhesive. Once hardened, a thermoset cannot be melted for reshaping, in contrast to thermoplastic polymers which are commonly produced and distributed in the form of pellets, and shaped into the final product for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermoplastic
A thermoplastic, or thermosoft plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling. Most thermoplastics have a high molecular weight. The polymer chains associate by intermolecular forces, which weaken rapidly with increased temperature, yielding a viscous liquid. In this state, thermoplastics may be reshaped and are typically used to produce parts by various polymer processing techniques such as injection molding, compression molding, calendering, and extrusion. Thermoplastics differ from thermosetting polymers (or "thermosets"), which form irreversible chemical bonds during the curing process. Thermosets do not melt when heated, but typically decompose and do not reform upon cooling. Above its glass transition temperature and below its melting point, the physical properties of a thermoplastic change drastically without an associated phase change. Some thermoplastics do not fully cry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Poly(methyl Methacrylate)
Poly(methyl methacrylate) (PMMA) belongs to a group of materials called engineering plastics. It is a transparent thermoplastic. PMMA is also known as acrylic, acrylic glass, as well as by the trade names and brands Crylux, Plexiglas, Acrylite, Astariglas, Lucite, Perclax, and Perspex, among several others ( see below). This plastic is often used in sheet form as a lightweight or shatter-resistant alternative to glass. It can also be used as a casting resin, in inks and coatings, and for many other purposes. Although not a type of familiar silica-based glass, the substance, like many thermoplastics, is often technically classified as a type of glass, in that it is a non-crystalline vitreous substance—hence its occasional historic designation as ''acrylic glass''. Chemically, it is the synthetic polymer of methyl methacrylate. It was developed in 1928 in several different laboratories by many chemists, such as William Chalmers, Otto Röhm, and Walter Bauer, and first brou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polycarbonate
Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, tough materials, and some grades are optically transparent. They are easily worked, molded, and thermoformed. Because of these properties, polycarbonates find many applications. Polycarbonates do not have a unique resin identification code (RIC) and are identified as "Other", 7 on the RIC list. Products made from polycarbonate can contain the precursor monomer bisphenol A (BPA). Structure Carbonate esters have planar OC(OC)2 cores, which confers rigidity. The unique O=C bond is short (1.173 Å in the depicted example), while the C-O bonds are more ether-like (the bond distances of 1.326 Å for the example depicted). Polycarbonates received their name because they are polymers containing carbonate groups (−O−(C=O)−O−). A balance of useful features, including temperature resistance, impact resistance and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It is a poor barrier to oxygen and water vapour and has a relatively low melting point. Polystyrene is one of the most widely used plastics, the scale of its production being several million tonnes per year. Polystyrene can be naturally transparent, but can be colored with colorants. Uses include protective packaging (such as packing peanuts and in the jewel cases used for storage of optical discs such as CDs and occasionally DVDs), containers, lids, bottles, trays, tumblers, disposable cutlery, in the making of models, and as an alternative material for phonograph records. As a thermoplastic polymer, polystyrene is in a solid (glassy) state at room temperature but flows if heated above about 100 °C, its glass transition temperature ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyethylene Terephthalate
Polyethylene terephthalate (or poly(ethylene terephthalate), PET, PETE, or the obsolete PETP or PET-P), is the most common thermoplastic polymer resin of the polyester family and is used in fibres for clothing, containers for liquids and foods, and thermoforming for manufacturing, and in combination with glass fibre for engineering resins. In 2016, annual production of PET was 56 million tons. The biggest application is in fibres (in excess of 60%), with bottle production accounting for about 30% of global demand. In the context of textile applications, PET is referred to by its common name, polyester, whereas the acronym ''PET'' is generally used in relation to packaging. Polyester makes up about 18% of world polymer production and is the fourth-most-produced polymer after polyethylene (PE), polypropylene (PP) and polyvinyl chloride (PVC). PET consists of repeating (C10H8O4) units. PET is commonly recycled, and has the digit 1 (♳) as its resin identification code (R ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |