|
IR-spectrum
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functional groups in solid, liquid, or gaseous forms. It can be used to characterize new materials or identify and verify known and unknown samples. The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer (or spectrophotometer) which produces an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance (or transmittance) on the vertical axis vs. frequency, wavenumber or wavelength on the horizontal axis. Typical units of wavenumber used in IR spectra are reciprocal centimeters, with the symbol cm−1. Units of IR wavelength are commonly given in micrometers (formerly called "microns"), symbol μm, which are related to the wavenumber in a reciprocal way. A commo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osiris-Rex Ovirs Gsfc 20150619 2015-12655 019-023
OSIRIS-REx (Origins, Spectral Interpretation, Resource Identification, Security, Regolith Explorer) is a NASA asteroid-study and sample-return mission. The mission's primary goal is to obtain a sample of at least from 101955 Bennu, a C-type asteroid, carbonaceous near-Earth object, near-Earth asteroid, and return the sample to Earth for a detailed analysis. The material returned is expected to enable scientists to learn more about the formation and evolution of the Solar System, its initial stages of planet formation, and the source of organic compounds that led to the Abiogenesis, formation of life on Earth. OSIRIS-REx was launched on 8 September 2016, flew past Earth on 22 September 2017, and rendezvoused with 101955 Bennu, Bennu on 3 December 2018. It spent the next several months analyzing the surface to find a suitable site from which to extract a sample. On 20 October 2020, OSIRIS-REx touched down on Bennu and successfully collected a sample. Though ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fourier Transform Infrared Spectroscopy
Fourier-transform infrared spectroscopy (FTIR) is a technique used to obtain an infrared spectrum of absorption or emission of a solid, liquid, or gas. An FTIR spectrometer simultaneously collects high-resolution spectral data over a wide spectral range. This confers a significant advantage over a dispersive spectrometer, which measures intensity over a narrow range of wavelengths at a time. The term ''Fourier-transform infrared spectroscopy'' originates from the fact that a Fourier transform (a mathematical process) is required to convert the raw data into the actual spectrum. Conceptual introduction The goal of absorption spectroscopy techniques (FTIR, ultraviolet-visible ("UV-vis") spectroscopy, etc.) is to measure how much light a sample absorbs at each wavelength. The most straightforward way to do this, the "dispersive spectroscopy" technique, is to shine a monochromatic light beam at a sample, measure how much of the light is absorbed, and repeat for each different ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
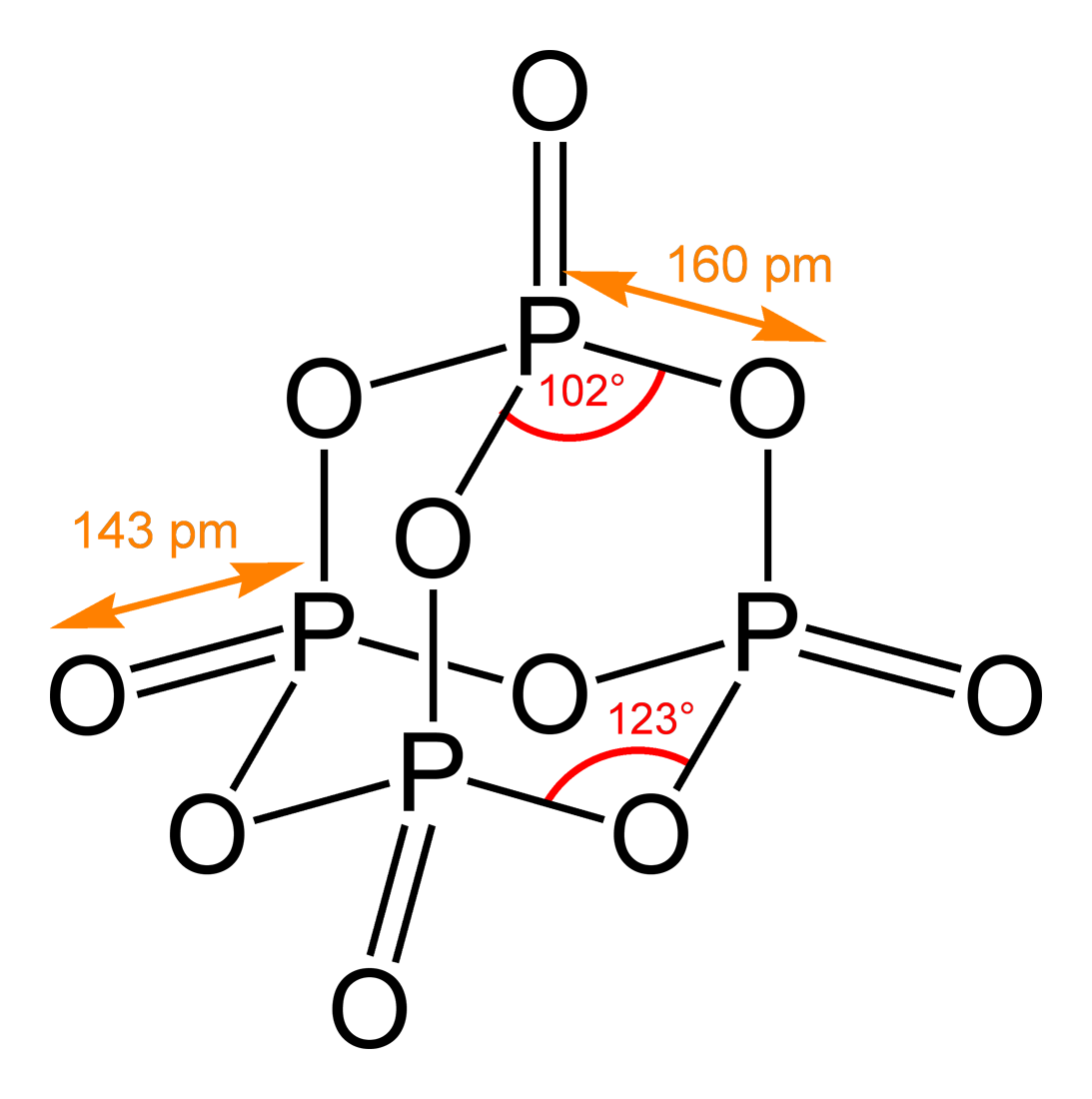
Chemical Structure
A chemical structure determination includes a chemist's specifying the molecular geometry and, when feasible and necessary, the electronic structure of the target molecule or other solid. Molecular geometry refers to the spatial arrangement of atoms in a molecule and the chemical bonds that hold the atoms together, and can be represented using structural formulae and by molecular models; complete electronic structure descriptions include specifying the occupation of a molecule's molecular orbitals. Structure determination can be applied to a range of targets from very simple molecules (e.g., diatomic oxygen or nitrogen), to very complex ones (e.g., such as protein or DNA). Background Theories of chemical structure were first developed by August Kekulé, Archibald Scott Couper, and Aleksandr Butlerov, among others, from about 1858. These theories were first to state that chemical compounds are not a random cluster of atoms and functional groups, but rather had a definite order ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromomethane IR Spectroscopy
Bromomethane, commonly known as methyl bromide, is an organobromine compound with formula C H3 Br. This colorless, odorless, nonflammable gas is produced both industrially and biologically. It has a tetrahedral shape and it is a recognized ozone-depleting chemical. It was used extensively as a pesticide until being phased out by most countries in the early 2000s. Occurrence and manufacture Bromomethane originates from both natural and human sources. In the ocean, marine organisms are estimated to produce 56,000 tonnes annually. It is also produced in small quantities by certain terrestrial plants, such as members of the family Brassicaceae. It is manufactured for agricultural and industrial use by treating methanol with bromine in the presence of sulfur or hydrogen sulfide: :6 CH3OH + 3 Br2 + S → 6 CH3Br + 2 H2O + H2SO4 Uses In 1999, an estimated 71,500 tonnes of synthetic methyl bromide were used annually worldwide. 97% of this estimate was used for fumigation purposes, w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terahertz Radiation
Terahertz radiation – also known as submillimeter radiation, terahertz waves, tremendously high frequency (THF), T-rays, T-waves, T-light, T-lux or THz – consists of electromagnetic waves within the ITU-designated band of frequencies from 0.3 to 3 terahertz (THz), although the upper boundary is somewhat arbitrary and is considered by some sources as 30 THz. One terahertz is 1012 Hz or 1000 GHz. Wavelengths of radiation in the terahertz band correspondingly range from 1 mm to 0.1 mm = 100 µm. Because terahertz radiation begins at a wavelength of around 1 millimeter and proceeds into shorter wavelengths, it is sometimes known as the ''submillimeter band'', and its radiation as ''submillimeter waves'', especially in astronomy. This band of electromagnetic radiation lies within the transition region between microwave and far infrared, and can be regarded as either. Terahertz radiation is strongly absorbed by the gases o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microwave
Microwave is a form of electromagnetic radiation with wavelengths ranging from about one meter to one millimeter corresponding to frequencies between 300 MHz and 300 GHz respectively. Different sources define different frequency ranges as microwaves; the above broad definition includes both UHF and EHF (millimeter wave) bands. A more common definition in radio-frequency engineering is the range between 1 and 100 GHz (wavelengths between 0.3 m and 3 mm). In all cases, microwaves include the entire SHF band (3 to 30 GHz, or 10 to 1 cm) at minimum. Frequencies in the microwave range are often referred to by their IEEE radar band designations: S, C, X, Ku, K, or Ka band, or by similar NATO or EU designations. The prefix ' in ''microwave'' is not meant to suggest a wavelength in the micrometer range. Rather, it indicates that microwaves are "small" (having shorter wavelengths), compared to the radio waves used prior to microwave te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rotational Spectroscopy
Rotational spectroscopy is concerned with the measurement of the energies of transitions between quantized rotational states of molecules in the gas phase. The spectra of polar molecules can be measured in absorption or emission by microwave spectroscopy or by far infrared spectroscopy. The rotational spectra of non-polar molecules cannot be observed by those methods, but can be observed and measured by Raman spectroscopy. Rotational spectroscopy is sometimes referred to as ''pure'' rotational spectroscopy to distinguish it from rotational-vibrational spectroscopy where changes in rotational energy occur together with changes in vibrational energy, and also from ro-vibronic spectroscopy (or just vibronic spectroscopy) where rotational, vibrational and electronic energy changes occur simultaneously. For rotational spectroscopy, molecules are classified according to symmetry into spherical top, linear and symmetric top; analytical expressions can be derived for the rotational ene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rotational–vibrational Spectroscopy
Rotational–vibrational spectroscopy is a branch of molecular spectroscopy concerned with infrared and Raman spectra of molecules in the gas phase. Transitions involving changes in both vibrational and rotational states can be abbreviated as rovibrational (or ro-vibrational) transitions. When such transitions emit or absorb photons (electromagnetic radiation), the frequency is proportional to the difference in energy levels and can be detected by certain kinds of spectroscopy. Since changes in rotational energy levels are typically much smaller than changes in vibrational energy levels, changes in rotational state are said to give fine structure to the vibrational spectrum. For a given vibrational transition, the same theoretical treatment as for pure rotational spectroscopy gives the rotational quantum numbers, energy levels, and selection rules. In linear and spherical top molecules, rotational lines are found as simple progressions at both higher and lower frequencies relat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
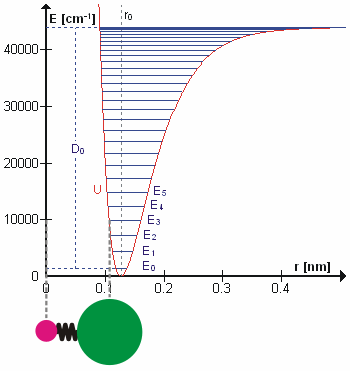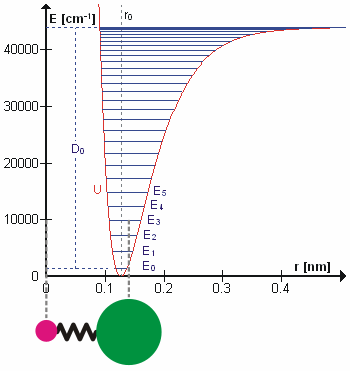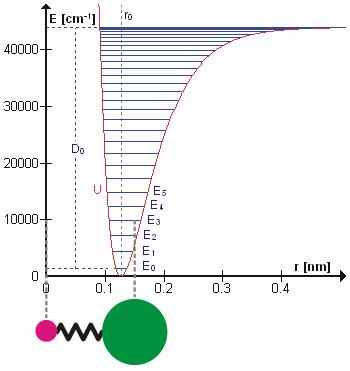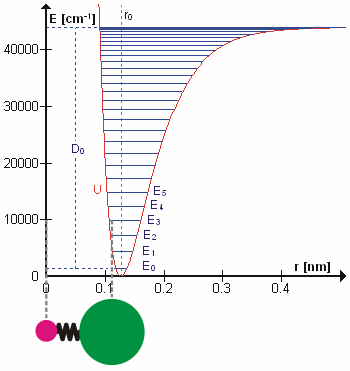
Molecular Vibration
A molecular vibration is a periodic motion of the atoms of a molecule relative to each other, such that the center of mass of the molecule remains unchanged. The typical vibrational frequencies range from less than 1013 Hz to approximately 1014 Hz, corresponding to wavenumbers of approximately 300 to 3000 cm−1 and wavelengths of approximately 30 to 3 µm. For a diatomic molecule A−B, the vibrational frequency in s−1 is given by \nu = \frac \sqrt , where k is the force constant in dyne/cm or erg/cm2 and μ is the reduced mass given by \frac = \frac+\frac. The vibrational wavenumber in cm−1 is \tilde \;= \frac \sqrt, where c is the speed of light in cm/s. Vibrations of polyatomic molecules are described in terms of normal modes, which are independent of each other, but each normal mode involves simultaneous vibrations of different parts of the molecule. In general, a non-linear molecule with ''N'' atoms has 3''N'' – 6 normal modes of vibration, but a ''linear'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Overtone Band
In vibrational spectroscopy, an overtone band is the spectral band that occurs in a vibrational spectrum of a molecule when the molecule makes a transition from the ground state (v=0) to the second excited state (v=2), where v is the vibrational quantum number (a non-negative integer) obtained from solving the Schrödinger equation for the molecule. Generally, in order to study the vibrational spectra of molecules, chemical bond vibrations are assumed to be approximable as simple harmonic oscillators. Thus a quadratic potential is used in the Schrödinger equation to solve for the vibrational energy eigenstates and their eigenvalues. These energy states are quantized, meaning they can assume only some "discrete" values of energy. When electromagnetic radiation is shined on a sample, the molecules can absorb energy from the radiation and change their vibrational energy state. However, the molecules can absorb energy from radiation only under certain condition, namely- there should b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Far Infrared
Far infrared (FIR) is a region in the infrared spectrum of electromagnetic radiation. Far infrared is often defined as any radiation with a wavelength of 15 micrometers (μm) to 1 mm (corresponding to a range of about 20 THz to 300 GHz), which places far infrared radiation within the CIE IR-B and IR-C bands. The long-wave side of the FIR spectrum overlaps with so named terahertz radiation. A.Glagoleva-Arkadiewa. (1924). "Short Electromagnetic Waves of wave-length up to 82 Microns". ''Nature'' 2844 113. do10.1038/113640a0/ref> Different sources use different boundaries for the far infrared; for example, astronomers sometimes define far infrared as wavelengths between 25 μm and 350 μm. Visible light includes radiation with wavelengths between 400 nm and 700 nm, meaning that far infrared photons have tens to hundreds of times less energy than visible light photons. Applications Astronomy Due to black-body radiation, objects with temper ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Near-infrared Spectroscopy
Near-infrared spectroscopy (NIRS) is a spectroscopic method that uses the near-infrared region of the electromagnetic spectrum (from 780 nm to 2500 nm). Typical applications include medical and physiological diagnostics and research including blood sugar, pulse oximetry, functional neuroimaging, sports medicine, elite sports training, ergonomics, rehabilitation, neonatal research, brain computer interface, urology (bladder contraction), and neurology (neurovascular coupling). There are also applications in other areas as well such as pharmaceutical, food and agrochemical quality control, atmospheric chemistry, combustion research and astronomy. Theory Near-infrared spectroscopy is based on molecular overtone and combination vibrations. Such transitions are forbidden by the selection rules of quantum mechanics. As a result, the molar absorptivity in the near-IR region is typically quite small. (NIR absorption bands are typically 10–100 times weaker than the correspond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)