Hydrogen Molecule on:
[Wikipedia]
[Google]
[Amazon]
Hydrogen is the
 The ground state
The ground state

 *
*
 Compounds of hydrogen are often called
Compounds of hydrogen are often called


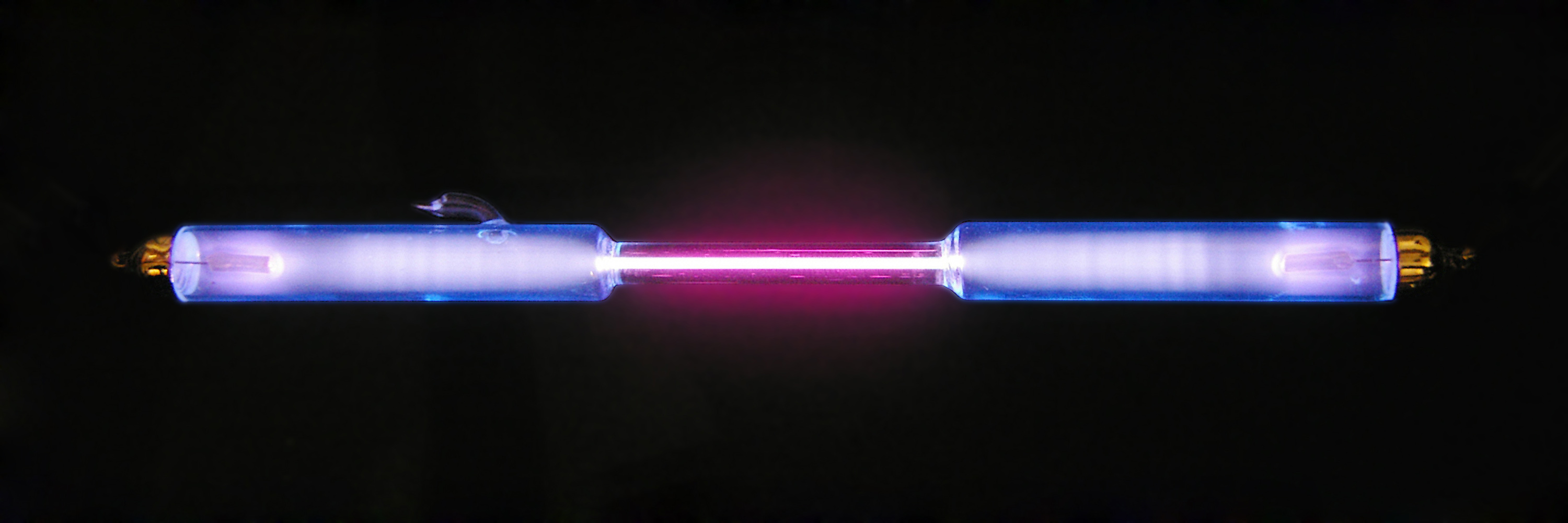 Hydrogen has three naturally occurring isotopes, denoted , and . Other, highly unstable nuclei ( to ) have been synthesized in the laboratory but not observed in nature.
* is the most common hydrogen isotope, with an abundance of more than 99.98%. Because the
Hydrogen has three naturally occurring isotopes, denoted , and . Other, highly unstable nuclei ( to ) have been synthesized in the laboratory but not observed in nature.
* is the most common hydrogen isotope, with an abundance of more than 99.98%. Because the
chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
with the symbol H and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
1. Hydrogen is the lightest element. At standard conditions
Standard temperature and pressure (STP) are standard sets of conditions for experimental measurements to be established to allow comparisons to be made between different sets of data. The most used standards are those of the International Union ...
hydrogen is a gas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or ...
of diatomic molecule
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear. O ...
s having the formula . It is colorless, odorless
The sense of smell, or olfaction, is the special sense through which smells (or odors) are perceived. The sense of smell has many functions, including detecting desirable foods, hazards, and pheromones, and plays a role in taste.
In humans, it ...
, taste
The gustatory system or sense of taste is the sensory system that is partially responsible for the perception of taste (flavor). Taste is the perception produced or stimulated when a substance in the mouth reacts chemically with taste receptor ...
less, non-toxic, and highly combustible
A combustible material is something that can burn (i.e., ''combust'') in air. A combustible material is flammable if it ignites easily at ambient temperatures. In other words, a combustible material ignites with some effort and a flammable mat ...
. Hydrogen is the most abundant chemical substance in the universe
The universe is all of space and time and their contents, including planets, stars, galaxies, and all other forms of matter and energy. The Big Bang theory is the prevailing cosmological description of the development of the universe. ...
, constituting roughly 75% of all normal Normal(s) or The Normal(s) may refer to:
Film and television
* ''Normal'' (2003 film), starring Jessica Lange and Tom Wilkinson
* ''Normal'' (2007 film), starring Carrie-Anne Moss, Kevin Zegers, Callum Keith Rennie, and Andrew Airlie
* ''Norma ...
matter
In classical physics and general chemistry, matter is any substance that has mass and takes up space by having volume. All everyday objects that can be touched are ultimately composed of atoms, which are made up of interacting subatomic part ...
.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter
Dark matter is a hypothetical form of matter thought to account for approximately 85% of the matter in the universe. Dark matter is called "dark" because it does not appear to interact with the electromagnetic field, which means it does not a ...
and dark energy
In physical cosmology and astronomy, dark energy is an unknown form of energy that affects the universe on the largest scales. The first observational evidence for its existence came from measurements of supernovas, which showed that the univ ...
. Stars such as the Sun
The Sun is the star at the center of the Solar System. It is a nearly perfect ball of hot plasma, heated to incandescence by nuclear fusion reactions in its core. The Sun radiates this energy mainly as light, ultraviolet, and infrared radi ...
are mainly composed of hydrogen in the . Most of the hydrogen on Earth exists in molecular forms such as water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
and organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. T ...
s. For the most common isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numb ...
of hydrogen (symbol 1H) each atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, ...
has one proton, one electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
, and no neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons beh ...
s.
In the early universe
The universe is all of space and time and their contents, including planets, stars, galaxies, and all other forms of matter and energy. The Big Bang theory is the prevailing cosmological description of the development of the universe. ...
, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 years later during the recombination epoch, when the plasma had cooled enough for electrons
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
to remain bound to protons.
Hydrogen is nonmetallic (except it becomes metallic
Metallic may be a reference to:
*Metal
* Metalloid, metal-like substance
*Metallic bonding, type of chemical bonding
* Metallicity, in astronomy the proportion of elements other than helium and hydrogen in an object
*Metallic color, a color that ...
at extremely high pressures) and readily forms a single covalent bond with most nonmetallic elements, forming compounds such as water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
and nearly all organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. T ...
s. Hydrogen plays a particularly important role in acid–base reaction
An acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH via titration. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their applica ...
s because these reactions usually involve the exchange of protons between soluble molecules. In ionic compounds, hydrogen can take the form of a negative charge (i.e., anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
) where it is known as a hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride ...
, or as a positively charged (i.e., cation) species
In biology, a species is the basic unit of classification and a taxonomic rank of an organism, as well as a unit of biodiversity. A species is often defined as the largest group of organisms in which any two individuals of the appropriate s ...
denoted by the symbol . The cation is simply a proton (symbol p) but its behavior in aqueous solutions and in ionic compounds involves screening
Screening may refer to:
* Screening cultures, a type a medical test that is done to find an infection
* Screening (economics), a strategy of combating adverse selection (includes sorting resumes to select employees)
* Screening (environmental), a ...
of its electric charge
Electric charge is the physical property of matter that causes charged matter to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative'' (commonly carried by protons and electrons respe ...
by nearby polar
Polar may refer to:
Geography
Polar may refer to:
* Geographical pole, either of two fixed points on the surface of a rotating body or planet, at 90 degrees from the equator, based on the axis around which a body rotates
* Polar climate, the c ...
molecules or anions. Because hydrogen is the only neutral atom for which the Schrödinger equation
The Schrödinger equation is a linear partial differential equation that governs the wave function of a quantum-mechanical system. It is a key result in quantum mechanics, and its discovery was a significant landmark in the development of th ...
can be solved analytically, the study of its energetics and chemical bonding has played a key role in the development of quantum mechanics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistr ...
.
Hydrogen gas was first artificially produced in the early 16th century by the reaction of acids on metals. In 1766–1781, Henry Cavendish
Henry Cavendish ( ; 10 October 1731 – 24 February 1810) was an English natural philosopher and scientist who was an important experimental and theoretical chemist and physicist. He is noted for his discovery of hydrogen, which he termed "infl ...
was the first to recognize that hydrogen gas was a discrete substance, and that it produces water when burned, the property for which it was later named: in Greek, hydrogen means "water-former".
Industrial production is mainly from steam reforming
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly natural gas is the feedstock. The main purpose of this technology is hydrogen product ...
of natural gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbo ...
, oil reforming, or coal gasification. A small percentage is also produced using more energy-intensive methods such as the electrolysis of water
Electrolysis of water, also known as electrochemical water splitting, is the process of using electricity to decompose water into oxygen and hydrogen gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, or remi ...
. Most hydrogen is used near the site of its production, the two largest uses being fossil fuel processing (e.g., hydrocracking) and ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous wa ...
production, mostly for the fertilizer market. It can be burned to produce heat or combined with oxygen in fuel cells to generate electricity directly, with water being the only emissions at the point of usage. Hydrogen atoms (but not gaseous molecules) are problematic in metallurgy because they can embrittle many metals.
Properties
Combustion
Hydrogen gas (dihydrogen or molecular hydrogen) is highly flammable: : (572 kJ/2 mol = 286 kJ/mol = 141.865 MJ/kg)286 kJ/mol: energy per mole of the combustible material (molecular hydrogen). Theenthalpy of combustion
The heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the amount of heat released during the combustion of a specified amount of it.
The ''calorific value'' is the total energy relea ...
is −286 kJ/mol.
Hydrogen gas forms explosive mixtures with air in concentrations from 4–74% and with chlorine at 5–95%. The explosive reactions may be triggered by spark, heat, or sunlight. The hydrogen autoignition temperature, the temperature of spontaneous ignition in air, is .
Flame
Pure hydrogen-oxygen flames emitultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation ...
light and with high oxygen mix are nearly invisible to the naked eye, as illustrated by the faint plume of the Space Shuttle Main Engine, compared to the highly visible plume of a Space Shuttle Solid Rocket Booster
The Space Shuttle Solid Rocket Booster (SRB) was the first solid-propellant rocket to be used for primary propulsion on a vehicle used for human spaceflight. A pair of these provided 85% of the Space Shuttle's thrust at liftoff and for the first ...
, which uses an ammonium perchlorate composite. The detection of a burning hydrogen leak may require a flame detector
A flame detector is a sensor designed to detect and respond to the presence of a flame or fire, allowing flame detection. Responses to a detected flame depend on the installation, but can include sounding an alarm, deactivating a fuel line (such as ...
; such leaks can be very dangerous. Hydrogen flames in other conditions are blue, resembling blue natural gas flames. The destruction of the Hindenburg airship was a notorious example of hydrogen combustion and the cause is still debated. The visible flames in the photographs were the result of carbon compounds in the airship skin burning.
Reactants
is unreactive compared to diatomic elements such ashalogens
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group i ...
or oxygen. The thermodynamic basis of this low reactivity is the very strong H–H bond, with a bond dissociation energy
The bond-dissociation energy (BDE, ''D''0, or ''DH°'') is one measure of the strength of a chemical bond . It can be defined as the standard enthalpy change when is cleaved by homolysis to give fragments A and B, which are usually radical ...
of 435.7 kJ/mol. The kinetic basis of the low reactivity is the nonpolar nature of and its weak polarizability. It spontaneously reacts with chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine i ...
and fluorine to form hydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride ga ...
and hydrogen fluoride, respectively. The reactivity of is strongly affected by the presence of metal catalysts. Thus, while mixtures of with or air combust readily when heated to at least 500 °C by a spark or flame, they do not react at room temperature in the absence of a catalyst.
Electron energy levels
energy level
A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical particles, which can have any amount of energy. The t ...
of the electron in a hydrogen atom is −13.6 eV, which is equivalent to an ultraviolet photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless, so they a ...
of roughly 91 nm wavelength.
The energy levels of hydrogen can be calculated fairly accurately using the Bohr model
In atomic physics, the Bohr model or Rutherford–Bohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electrons—similar to the structure of the Solar Syst ...
of the atom, which conceptualizes the electron as "orbiting" the proton in analogy to the Earth's orbit of the Sun. However, the atomic electron and proton are held together by electromagnetic force
In physics, electromagnetism is an interaction that occurs between particles with electric charge. It is the second-strongest of the four fundamental interactions, after the strong force, and it is the dominant force in the interactions o ...
, while planets and celestial objects are held by gravity
In physics, gravity () is a fundamental interaction which causes mutual attraction between all things with mass or energy. Gravity is, by far, the weakest of the four fundamental interactions, approximately 1038 times weaker than the stro ...
. Because of the discretization of angular momentum
In physics, angular momentum (rarely, moment of momentum or rotational momentum) is the rotational analog of linear momentum. It is an important physical quantity because it is a conserved quantity—the total angular momentum of a closed syst ...
postulated in early quantum mechanics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistr ...
by Bohr, the electron in the Bohr model can only occupy certain allowed distances from the proton, and therefore only certain allowed energies.
A more accurate description of the hydrogen atom comes from a purely quantum mechanical treatment that uses the Schrödinger equation
The Schrödinger equation is a linear partial differential equation that governs the wave function of a quantum-mechanical system. It is a key result in quantum mechanics, and its discovery was a significant landmark in the development of th ...
, Dirac equation
In particle physics, the Dirac equation is a relativistic wave equation derived by British physicist Paul Dirac in 1928. In its free form, or including electromagnetic interactions, it describes all spin- massive particles, called "Dirac par ...
or Feynman
Richard Phillips Feynman (; May 11, 1918 – February 15, 1988) was an American theoretical physicist, known for his work in the path integral formulation of quantum mechanics, the theory of quantum electrodynamics, the physics of the superflu ...
path integral formulation
The path integral formulation is a description in quantum mechanics that generalizes the action principle of classical mechanics. It replaces the classical notion of a single, unique classical trajectory for a system with a sum, or functional i ...
to calculate the probability density
In probability theory, a probability density function (PDF), or density of a continuous random variable, is a function whose value at any given sample (or point) in the sample space (the set of possible values taken by the random variable) can ...
of the electron around the proton. The most complicated treatments allow for the small effects of special relativity
In physics, the special theory of relativity, or special relativity for short, is a scientific theory regarding the relationship between space and time. In Albert Einstein's original treatment, the theory is based on two postulates:
# The laws ...
and vacuum polarization
In quantum field theory, and specifically quantum electrodynamics, vacuum polarization describes a process in which a background electromagnetic field produces virtual electron–positron pairs that change the distribution of charges and curr ...
. In the quantum mechanical treatment, the electron in a ground state hydrogen atom has no angular momentum at all—illustrating how the "planetary orbit" differs from electron motion.
Spin isomers
Molecular exists as two spin isomers, i.e. compounds that differ only in the spin states of their nuclei. In the orthohydrogen form, the spins of the two nuclei are parallel, forming a spintriplet state
In quantum mechanics, a triplet is a quantum state of a system with a spin of quantum number =1, such that there are three allowed values of the spin component, = −1, 0, and +1.
Spin, in the context of quantum mechanics, is not a mechanical r ...
having a total molecular spin ; in the parahydrogen form the spins are antiparallel and form a spin singlet state
In quantum mechanics, a singlet state usually refers to a system in which all electrons are paired. The term 'singlet' originally meant a linked set of particles whose net angular momentum is zero, that is, whose overall spin quantum number s=0. A ...
having spin . The equilibrium ratio of ortho- to para-hydrogen depends on temperature. At room temperature or warmer, equilibrium hydrogen gas contains about 25% of the para form and 75% of the ortho form. The ortho form is an excited state, having higher energy than the para form by 1.455 kJ/mol, and it converts to the para form over the course of several minutes when cooled to low temperature. The thermal properties of the forms differ because they differ in their allowed rotational quantum states, resulting in different thermal properties such as the heat capacity.
The ortho-to-para ratio in is an important consideration in the liquefaction and storage of liquid hydrogen
Liquid hydrogen (LH2 or LH2) is the liquid state of the element hydrogen. Hydrogen is found naturally in the molecular H2 form.
To exist as a liquid, H2 must be cooled below its critical point of 33 K. However, for it to be in a fully l ...
: the conversion from ortho to para is exothermic and produces enough heat to evaporate a most of the liquid if not converted first to parahydrogen during the cooling process. Catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
s for the ortho-para interconversion, such as ferric oxide
Iron(III) oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron(II) oxide (FeO), which is rare; and iron(II,III) oxide (Fe3O4), which also occurs naturall ...
and activated carbon compounds, are used during hydrogen cooling to avoid this loss of liquid.
Phases

 *
* Gas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or ...
eous hydrogen
* Liquid hydrogen
Liquid hydrogen (LH2 or LH2) is the liquid state of the element hydrogen. Hydrogen is found naturally in the molecular H2 form.
To exist as a liquid, H2 must be cooled below its critical point of 33 K. However, for it to be in a fully l ...
* Slush hydrogen Slush hydrogen is a combination of liquid hydrogen and solid hydrogen at the triple point with a lower temperature and a higher density than liquid hydrogen. It is commonly formed by repeating a freeze-thaw process. This is most easily done by bring ...
* Solid hydrogen
Solid hydrogen is the solid state of the element hydrogen, achieved by decreasing the temperature below hydrogen's melting point of . It was collected for the first time by James Dewar in 1899 and published with the title "Sur la solidification de ...
* Metallic hydrogen
Metallic hydrogen is a phase of hydrogen in which it behaves like an electrical conductor. This phase was predicted in 1935 on theoretical grounds by Eugene Wigner and Hillard Bell Huntington.
At high pressure and temperatures, metallic hydroge ...
* Plasma hydrogen
Compounds
Covalent and organic compounds
While is not very reactive under standard conditions, it does form compounds with most elements. Hydrogen can form compounds with elements that are moreelectronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
, such as halogens (F, Cl, Br, I), or oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
; in these compounds hydrogen takes on a partial positive charge. When bonded to a more electronegative element, particularly fluorine, oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
, or nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
, hydrogen can participate in a form of medium-strength noncovalent bonding with another electronegative element with a lone pair, a phenomenon called hydrogen bonding that is critical to the stability of many biological molecules. Hydrogen also forms compounds with less electronegative elements, such as metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typicall ...
s and metalloids, where it takes on a partial negative charge. These compounds are often known as hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride ...
s.
Hydrogen forms a vast array of compounds with carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
called the hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
s, and an even vaster array with heteroatoms that, because of their general association with living things, are called organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. T ...
s. The study of their properties is known as organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, ...
and their study in the context of living organism
In biology, an organism () is any living system that functions as an individual entity. All organisms are composed of cells (cell theory). Organisms are classified by taxonomy into groups such as multicellular animals, plants, and ...
s is known as biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and ...
. By some definitions, "organic" compounds are only required to contain carbon. However, most of them also contain hydrogen, and because it is the carbon-hydrogen bond that gives this class of compounds most of its particular chemical characteristics, carbon-hydrogen bonds are required in some definitions of the word "organic" in chemistry. Millions of hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
s are known, and they are usually formed by complicated pathways that seldom involve elemental hydrogen.
Hydrogen is highly soluble in many rare earth and transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that ca ...
s and is soluble in both nanocrystalline and amorphous metal
An amorphous metal (also known as metallic glass, glassy metal, or shiny metal) is a solid metallic material, usually an alloy, with disordered atomic-scale structure. Most metals are crystalline in their solid state, which means they have a high ...
s. Hydrogen solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solub ...
in metals is influenced by local distortions or impurities in the crystal lattice. These properties may be useful when hydrogen is purified by passage through hot palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself na ...
disks, but the gas's high solubility is a metallurgical problem, contributing to the embrittlement
Embrittlement is a significant decrease of ductility of a material, which makes the material brittle. Embrittlement is used to describe any phenomena where the environment compromises a stressed material's mechanical performance, such as temperatu ...
of many metals, complicating the design of pipelines and storage tanks.
Hydrides
 Compounds of hydrogen are often called
Compounds of hydrogen are often called hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride ...
s, a term that is used fairly loosely. The term "hydride" suggests that the H atom has acquired a negative or anionic character, denoted , and is used when hydrogen forms a compound with a more electropositive
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
element. The existence of the hydride anion
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of ...
, suggested by Gilbert N. Lewis in 1916 for group 1 and 2 salt-like hydrides, was demonstrated by Moers in 1920 by the electrolysis of molten lithium hydride
Lithium hydride is an inorganic compound with the formula Li H. This alkali metal hydride is a colorless solid, although commercial samples are grey. Characteristic of a salt-like (ionic) hydride, it has a high melting point, and it is not solub ...
(LiH), producing a stoichiometric
Stoichiometry refers to the relationship between the quantities of reactants and products before, during, and following chemical reactions.
Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equ ...
quantity of hydrogen at the anode. For hydrides other than group 1 and 2 metals, the term is quite misleading, considering the low electronegativity of hydrogen. An exception in group 2 hydrides is , which is polymeric. In lithium aluminium hydride, the anion carries hydridic centers firmly attached to the Al(III).
Although hydrides can be formed with almost all main-group elements, the number and combination of possible compounds varies widely; for example, more than 100 binary borane hydrides are known, but only one binary aluminium hydride. Binary indium
Indium is a chemical element with the symbol In and atomic number 49. Indium is the softest metal that is not an alkali metal. It is a silvery-white metal that resembles tin in appearance. It is a post-transition metal that makes up 0.21 parts ...
hydride has not yet been identified, although larger complexes exist.
In inorganic chemistry, hydrides can also serve as bridging ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually ...
s that link two metal centers in a coordination complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as '' ligands'' or complexing agents. ...
. This function is particularly common in group 13 element
The Group 13 network ( pl, Trzynastka, Yiddish: ''דאָס דרײַצענטל'') was a Jewish Nazi collaborationist organization in the Warsaw Ghetto during the German occupation of Poland in World War II. The rise and fall of the Group ...
s, especially in borane
Trihydridoboron, also known as borane or borine, is an unstable and highly reactive molecule with the chemical formula . The preparation of borane carbonyl, BH3(CO), played an important role in exploring the chemistry of boranes, as it indicated ...
s ( boron hydrides) and aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
complexes, as well as in clustered carborane
Carboranes are electron-delocalized (non-classically bonded) clusters composed of boron, carbon and hydrogen atoms.Grimes, R. N., ''Carboranes 3rd Ed.'', Elsevier, Amsterdam and New York (2016), . Like many of the related boron hydrides, these c ...
s.
Protons and acids
Oxidation of hydrogen removes its electron and gives , which contains no electrons and anucleus
Nucleus ( : nuclei) is a Latin word for the seed inside a fruit. It most often refers to:
*Atomic nucleus, the very dense central region of an atom
* Cell nucleus, a central organelle of a eukaryotic cell, containing most of the cell's DNA
Nucl ...
which is usually composed of one proton. That is why is often called a proton. This species is central to discussion of acids. Under the Brønsted–Lowry acid–base theory
The Brønsted–Lowry theory (also called proton theory of acids and bases) is an acid–base reaction theory which was proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923. The fundamental concept of this the ...
, acids are proton donors, while bases are proton acceptors.
A bare proton, , cannot exist in solution or in ionic crystals because of its unstoppable attraction to other atoms or molecules with electrons. Except at the high temperatures associated with plasmas, such protons cannot be removed from the electron cloud
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any sp ...
s of atoms and molecules, and will remain attached to them. However, the term 'proton' is sometimes used loosely and metaphorically to refer to positively charged or cationic hydrogen attached to other species in this fashion, and as such is denoted "" without any implication that any single protons exist freely as a species.
To avoid the implication of the naked "solvated proton" in solution, acidic aqueous solutions are sometimes considered to contain a less unlikely fictitious species, termed the "hydronium
In chemistry, hydronium (hydroxonium in traditional British English) is the common name for the aqueous cation , the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid i ...
ion" (). However, even in this case, such solvated hydrogen cations are more realistically conceived as being organized into clusters that form species closer to . Other oxonium ion
In chemistry, an oxonium ion is any cation containing an oxygen atom that has three bonds and 1+ formal charge. The simplest oxonium ion is the hydronium ion ().
Alkyloxonium
Hydronium is one of a series of oxonium ions with the formula R''n' ...
s are found when water is in acidic solution with other solvents.
Although exotic on Earth, one of the most common ions in the universe is the ion, known as protonated molecular hydrogen or the trihydrogen cation.
Isotopes


 Hydrogen has three naturally occurring isotopes, denoted , and . Other, highly unstable nuclei ( to ) have been synthesized in the laboratory but not observed in nature.
* is the most common hydrogen isotope, with an abundance of more than 99.98%. Because the
Hydrogen has three naturally occurring isotopes, denoted , and . Other, highly unstable nuclei ( to ) have been synthesized in the laboratory but not observed in nature.
* is the most common hydrogen isotope, with an abundance of more than 99.98%. Because the nucleus
Nucleus ( : nuclei) is a Latin word for the seed inside a fruit. It most often refers to:
*Atomic nucleus, the very dense central region of an atom
* Cell nucleus, a central organelle of a eukaryotic cell, containing most of the cell's DNA
Nucl ...
of this isotope consists of only a single proton, it is given the descriptive but rarely used formal name '' protium''. It is unique among all stable isotopes in having no neutrons; see diproton
Although there are nine known isotopes of helium (2He) ( standard atomic weight: ), only helium-3 () and helium-4 () are stable. All radioisotopes are short-lived, the longest-lived being with a half-life of . The least stable is , with a half- ...
for a discussion of why others do not exist.
* , the other stable hydrogen isotope, is known as ''deuterium
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two stable isotopes of hydrogen (the other being protium, or hydrogen-1). The nucleus of a deuterium atom, called a deuteron, contains one proton and one ...
'' and contains one proton and one neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons beh ...
in the nucleus. All deuterium in the universe is thought to have been produced at the time of the Big Bang, and has endured since that time. Deuterium is not radioactive, and does not represent a significant toxicity hazard. Water enriched in molecules that include deuterium instead of normal hydrogen is called heavy water. Deuterium and its compounds are used as a non-radioactive label in chemical experiments and in solvents for -NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic fie ...
. Heavy water is used as a neutron moderator and coolant for nuclear reactors. Deuterium is also a potential fuel for commercial nuclear fusion
Nuclear fusion is a reaction in which two or more atomic nuclei are combined to form one or more different atomic nuclei and subatomic particles ( neutrons or protons). The difference in mass between the reactants and products is manife ...
.
* is known as ''tritium
Tritium ( or , ) or hydrogen-3 (symbol T or H) is a rare and radioactive isotope of hydrogen with half-life about 12 years. The nucleus of tritium (t, sometimes called a ''triton'') contains one proton and two neutrons, whereas the nucleus of ...
'' and contains one proton and two neutrons in its nucleus. It is radioactive, decaying into helium-3 through beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
with a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
of 12.32 years. It is so radioactive that it can be used in luminous paint
Luminous paint or luminescent paint is paint that exhibits luminescence. In other words, it gives off visible light through fluorescence, phosphorescence, or radioluminescence. There are three types of luminous paints: fluorescent paint, phosp ...
, making it useful in such things as watches. The glass prevents the small amount of radiation from getting out. Small amounts of tritium are produced naturally by the interaction of cosmic rays with atmospheric gases; tritium has also been released during nuclear weapons tests
Nuclear weapons tests are experiments carried out to determine nuclear weapons' effectiveness, yield, and explosive capability. Testing nuclear weapons offers practical information about how the weapons function, how detonations are affected b ...
. It is used in nuclear fusion reactions, as a tracer in isotope geochemistry
Isotope geochemistry is an aspect of geology based upon the study of natural variations in the relative abundances of isotopes of various elements. Variations in isotopic abundance are measured by isotope ratio mass spectrometry, and can reveal ...
, and in specialized self-powered lighting
Tritium radioluminescence is the use of gaseous tritium, a radioactive isotope of hydrogen, to create visible light. Tritium emits electrons through beta decay and, when they interact with a phosphor material, light is emitted through the proces ...
devices. Tritium has also been used in chemical and biological labeling experiments as a radiolabel
A radioactive tracer, radiotracer, or radioactive label is a chemical compound in which one or more atoms have been replaced by a radionuclide so by virtue of its radioactive decay it can be used to explore the mechanism of chemical reactions by tr ...
.
Unique among the elements, distinct names are assigned to its isotopes in common use today. During the early study of radioactivity, various heavy radioactive isotopes were given their own names, but such names are no longer used, except for deuterium and tritium. The symbols D and T (instead of and ) are sometimes used for deuterium and tritium, but the symbol P is already in use for phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ear ...
and thus is not available for protium. In its nomenclatural
Nomenclature (, ) is a system of names or terms, or the rules for forming these terms in a particular field of arts or sciences. The principles of naming vary from the relatively informal conventions of everyday speech to the internationally ag ...
guidelines, the International Union of Pure and Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
(IUPAC) allows any of D, T, , and to be used, although and are preferred.
The exotic atom
An exotic atom is an otherwise normal atom in which one or more sub-atomic particles have been replaced by other particles of the same charge. For example, electrons may be replaced by other negatively charged particles such as muons (muonic atoms ...
muonium
Muonium is an exotic atom made up of an antimuon and an electron,
which was discovered in 1960 by Vernon W. Hughes
and is given the chemical symbol Mu. During the muon's lifetime, muonium can undergo chemical reactions. Due to the mass diffe ...
(symbol Mu), composed of an antimuon
A muon ( ; from the Greek letter mu (μ) used to represent it) is an elementary particle similar to the electron, with an electric charge of −1 '' e'' and a spin of , but with a much greater mass. It is classified as a lepton. As wit ...
and an electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
, can also be considered a light radioisotope of hydrogen. Because muons decay with lifetime , muonium is too unstable to exhibit observable chemistry. Nevertheless, muonium compounds are important test cases for quantum simulation, due to the mass difference between the antimuon and the proton, and IUPAC nomenclature incorporates such hypothetical compounds as muonium chloride (MuCl) and sodium muonide (NaMu), analogous to hydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride ga ...
and sodium hydride
Sodium hydride is the chemical compound with the empirical formula Na H. This alkali metal hydride is primarily used as a strong yet combustible base in organic synthesis. NaH is a saline (salt-like) hydride, composed of Na+ and H− ions, in co ...
respectively.
Thermal and physical properties
Table of thermal and physical properties of hydrogen (H2) at atmospheric pressure:History
Discovery and use
In 1671,Robert Boyle
Robert Boyle (; 25 January 1627 – 31 December 1691) was an Anglo-Irish natural philosopher, chemist, physicist, alchemist and inventor. Boyle is largely regarded today as the first modern chemist, and therefore one of the founders of ...
discovered and described the reaction between iron
Iron () is a chemical element with Symbol (chemistry), symbol Fe (from la, Wikt:ferrum, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 element, group 8 of the periodic table. It is, Abundanc ...
filings and dilute acids, which results in the production of hydrogen gas.
In 1766, Henry Cavendish
Henry Cavendish ( ; 10 October 1731 – 24 February 1810) was an English natural philosopher and scientist who was an important experimental and theoretical chemist and physicist. He is noted for his discovery of hydrogen, which he termed "infl ...
was the first to recognize hydrogen gas as a discrete substance, by naming the gas from a metal-acid reaction "inflammable air". He speculated that "inflammable air" was in fact identical to the hypothetical substance called "phlogiston
The phlogiston theory is a superseded scientific theory that postulated the existence of a fire-like element called phlogiston () contained within combustible bodies and released during combustion. The name comes from the Ancient Greek (''burni ...
" and further finding in 1781 that the gas produces water when burned. He is usually given credit for the discovery of hydrogen as an element. In 1783, Antoine Lavoisier
Antoine-Laurent de Lavoisier ( , ; ; 26 August 17438 May 1794),
CNRS (
CNRS (
Laplace reproduced Cavendish's finding that water is produced when hydrogen is burned.
Lavoisier produced hydrogen for his experiments on mass conservation by reacting a flux of steam with metallic
 Because of its simple atomic structure, consisting only of a proton and an electron, the hydrogen atom, together with the spectrum of light produced from it or absorbed by it, has been central to the development of the theory of
Because of its simple atomic structure, consisting only of a proton and an electron, the hydrogen atom, together with the spectrum of light produced from it or absorbed by it, has been central to the development of the theory of
 Hydrogen, as atomic H, is the most abundant
Hydrogen, as atomic H, is the most abundant
 The
The
 Hydrogen production using natural gas methane
Hydrogen production using natural gas methane
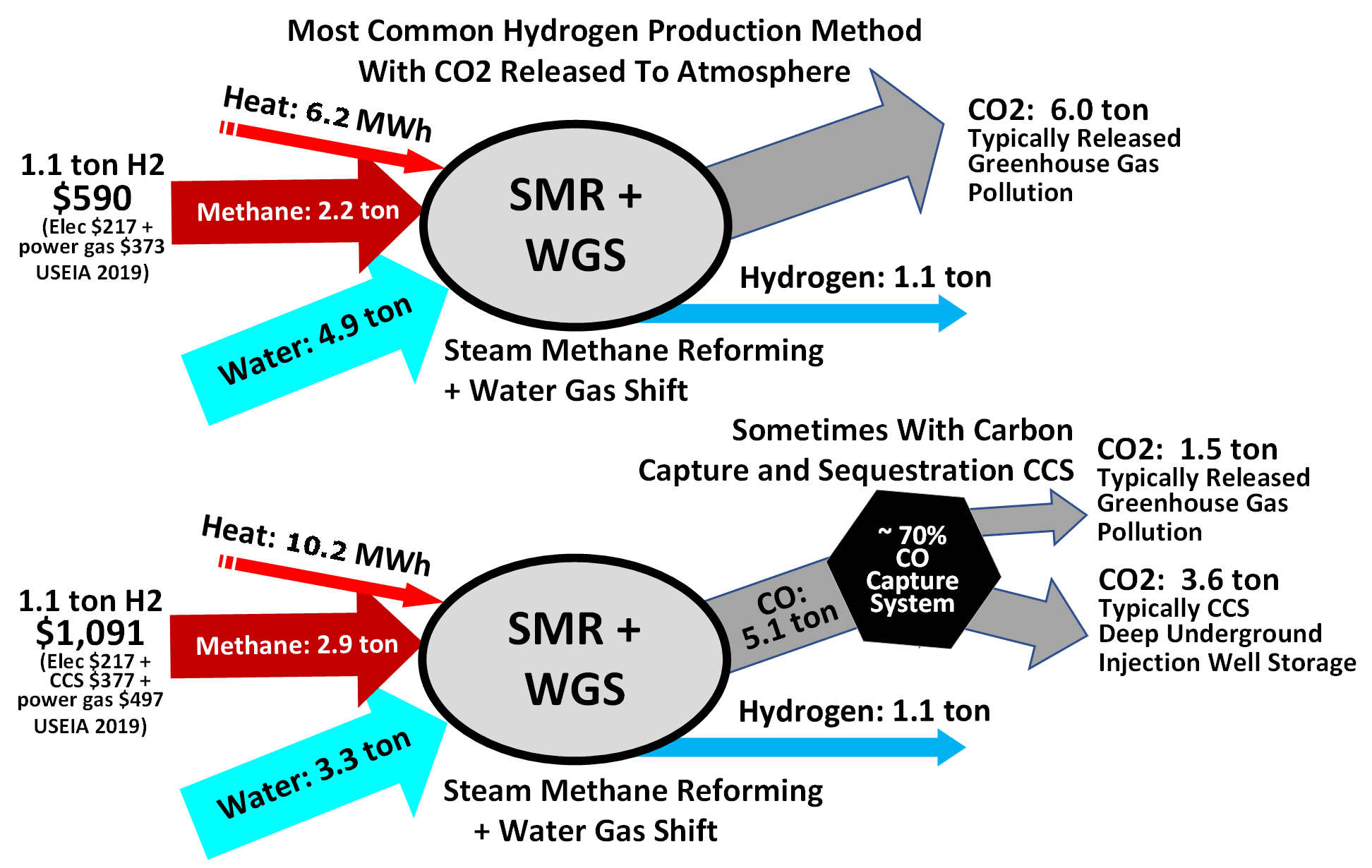 Hydrogen is often produced by reacting water with
Hydrogen is often produced by reacting water with
''carrier'' of energy rather than an energy resource, because there is no naturally occurring source of hydrogen in useful quantities.
Hydrogen can be burned to produce heat or combined with oxygen in fuel cells to generate electricity directly, with water being the only emissions at the point of usage. The overall lifecycle emissions of hydrogen depend on how it is produced. Nearly all of the world's current supply of hydrogen is created from fossil fuels. The main method is steam methane reforming, in which hydrogen is produced from a chemical reaction between steam and
Basic Hydrogen Calculations of Quantum Mechanics
at ''
{{Authority control Chemical elements Reactive nonmetals Diatomic nonmetals Nuclear fusion fuels Airship technology Reducing agents Refrigerants Gaseous signaling molecules E-number additives
iron
Iron () is a chemical element with Symbol (chemistry), symbol Fe (from la, Wikt:ferrum, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 element, group 8 of the periodic table. It is, Abundanc ...
through an incandescent iron tube heated in a fire. Anaerobic oxidation of iron by the protons of water at high temperature can be schematically represented by the set of following reactions:
:1)
:2)
:3)
Many metals such as zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name ''zirconium'' is taken from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian '' zargun'' (zircon; ''zar-gun'' ...
undergo a similar reaction with water leading to the production of hydrogen.
Hydrogen was liquefied for the first time by James Dewar
Sir James Dewar (20 September 1842 – 27 March 1923) was a British chemist and physicist. He is best known for his invention of the vacuum flask, which he used in conjunction with research into the liquefaction of gases. He also studied a ...
in 1898 by using regenerative cooling
Regenerative cooling is a method of cooling gases in which compressed gas is cooled by allowing it to expand and thereby take heat from the surroundings. The cooled expanded gas then passes through a heat exchanger where it cools the incoming comp ...
and his invention, the vacuum flask
A vacuum flask (also known as a Dewar flask, Dewar bottle or thermos) is an insulating storage vessel that greatly lengthens the time over which its contents remain hotter or cooler than the flask's surroundings. Invented by Sir James Dewa ...
. He produced solid hydrogen
Solid hydrogen is the solid state of the element hydrogen, achieved by decreasing the temperature below hydrogen's melting point of . It was collected for the first time by James Dewar in 1899 and published with the title "Sur la solidification de ...
the next year. Deuterium
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two stable isotopes of hydrogen (the other being protium, or hydrogen-1). The nucleus of a deuterium atom, called a deuteron, contains one proton and one ...
was discovered in December 1931 by Harold Urey
Harold Clayton Urey ( ; April 29, 1893 – January 5, 1981) was an American physical chemist whose pioneering work on isotopes earned him the Nobel Prize in Chemistry in 1934 for the discovery of deuterium. He played a significant role in th ...
, and tritium
Tritium ( or , ) or hydrogen-3 (symbol T or H) is a rare and radioactive isotope of hydrogen with half-life about 12 years. The nucleus of tritium (t, sometimes called a ''triton'') contains one proton and two neutrons, whereas the nucleus of ...
was prepared in 1934 by Ernest Rutherford
Ernest Rutherford, 1st Baron Rutherford of Nelson, (30 August 1871 – 19 October 1937) was a New Zealand physicist who came to be known as the father of nuclear physics.
''Encyclopædia Britannica'' considers him to be the greatest ...
, Mark Oliphant
Sir Marcus Laurence Elwin Oliphant, (8 October 1901 – 14 July 2000) was an Australian physicist and humanitarian who played an important role in the first experimental demonstration of nuclear fusion and in the development of nuclear weapon ...
, and Paul Harteck
Paul Karl Maria Harteck (20 July 190222 January 1985) was an Austrian physical chemist. In 1945 under Operation Epsilon in "the big sweep" throughout Germany, Harteck was arrested by the allied British and American Armed Forces for suspicion of ...
. Heavy water, which consists of deuterium in the place of regular hydrogen, was discovered by Urey's group in 1932. François Isaac de Rivaz
François Isaac de Rivaz (Paris, December 19, 1752 – Sion, July 30, 1828) was a French-born Swiss inventor and a politician. He invented a hydrogen-powered internal combustion engine with electric ignition and described it in a French paten ...
built the first de Rivaz engine The de Rivaz engine was a pioneering reciprocating engine designed and developed from 1804 by the Franco-Swiss inventor Isaac de Rivaz. The engine has a claim to be the world's first internal combustion engine and contained some features of modern ...
, an internal combustion engine powered by a mixture of hydrogen and oxygen in 1806. Edward Daniel Clarke
Edward Daniel Clarke (5 June 17699 March 1822) was an English clergyman, naturalist, mineralogist, and traveller.
Life
Edward Daniel Clarke was born at Willingdon, Sussex, and educated first at Uckfield School"Anthony Saunders, D.D." in Ma ...
invented the hydrogen gas blowpipe in 1819. The Döbereiner's lamp
Döbereiner's lamp, also called a "tinderbox" ("Feuerzeug"), is a lighter invented in 1823 by the German chemist Johann Wolfgang Döbereiner. The lighter is based on the Fürstenberger lighter (invented in Basel in 1780; in which hydrogen gas is ...
and limelight were invented in 1823.
The first hydrogen-filled balloon
A balloon is a flexible bag that can be inflated with a gas, such as helium, hydrogen, nitrous oxide, oxygen, and air. For special tasks, balloons can be filled with smoke, liquid water, granular media (e.g. sand, flour or rice), or light so ...
was invented by Jacques Charles
Jacques Alexandre César Charles (November 12, 1746 – April 7, 1823) was a French inventor, scientist, mathematician, and balloonist.
Charles wrote almost nothing about mathematics, and most of what has been credited to him was due to mistaking ...
in 1783. Hydrogen provided the lift for the first reliable form of air-travel following the 1852 invention of the first hydrogen-lifted airship by Henri Giffard
Baptiste Jules Henri Jacques Giffard (8 February 182514 April 1882) was a French engineer. In 1852 he invented the steam injector and the powered Giffard dirigible airship.
Career
Giffard was born in Paris in 1825. He invented the injector a ...
. German count Ferdinand von Zeppelin
Count Ferdinand von Zeppelin (german: Ferdinand Adolf Heinrich August Graf von Zeppelin; 8 July 1838 – 8 March 1917) was a German general and later inventor of the Zeppelin rigid airships. His name soon became synonymous with airships a ...
promoted the idea of rigid airships lifted by hydrogen that later were called Zeppelin
A Zeppelin is a type of rigid airship named after the German inventor Count Ferdinand von Zeppelin () who pioneered rigid airship development at the beginning of the 20th century. Zeppelin's notions were first formulated in 1874Eckener 1938, pp ...
s; the first of which had its maiden flight in 1900. Regularly scheduled flights started in 1910 and by the outbreak of World War I in August 1914, they had carried 35,000 passengers without a serious incident. Hydrogen-lifted airships were used as observation platforms and bombers during the war.
The first non-stop transatlantic crossing was made by the British airship ''R34 R34 may refer to:
* R34 (New York City Subway car)
* R34 (South Africa)
* HM Airship ''R.34'', a rigid airship of the Royal Air Force
* , a destroyer of the Royal Navy
* Nissan Skyline (R34), a mid-size car
* Nissan Skyline GT-R (R34), a sports ca ...
'' in 1919. Regular passenger service resumed in the 1920s and the discovery of helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
reserves in the United States promised increased safety, but the U.S. government refused to sell the gas for this purpose. Therefore, was used in the ''Hindenburg'' airship, which was destroyed in a midair fire over New Jersey
New Jersey is a state in the Mid-Atlantic and Northeastern regions of the United States. It is bordered on the north and east by the state of New York; on the east, southeast, and south by the Atlantic Ocean; on the west by the Delaware ...
on 6 May 1937. The incident was broadcast live on radio and filmed. Ignition of leaking hydrogen is widely assumed to be the cause, but later investigations pointed to the ignition of the aluminized
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ...
fabric coating by static electricity. But the damage to hydrogen's reputation as a lifting gas
A lifting gas or lighter-than-air gas is a gas that has a density lower than normal atmospheric gases and rises above them as a result. It is required for aerostats to create buoyancy, particularly in lighter-than-air aircraft, which include ballo ...
was already done and commercial hydrogen airship travel ceased. Hydrogen is still used, in preference to non-flammable but more expensive helium, as a lifting gas for weather balloons
A weather balloon, also known as sounding balloon, is a balloon (specifically a type of high-altitude balloon) that carries instruments aloft to send back information on atmospheric pressure, temperature, humidity and wind speed by means of a ...
.
In the same year, the first hydrogen-cooled turbogenerator
A hydrogen-cooled turbo generator is a turbo generator with gaseous hydrogen as a coolant. Hydrogen-cooled turbo generators are designed to provide a low- drag atmosphere and cooling for single-shaft and combined-cycle applications in combination ...
went into service with gaseous hydrogen as a coolant
A coolant is a substance, typically liquid, that is used to reduce or regulate the temperature of a system. An ideal coolant has high thermal capacity, low viscosity, is low-cost, non-toxic, chemically inert and neither causes nor promotes corrosi ...
in the rotor and the stator in 1937 at Dayton
Dayton () is the sixth-largest city in the U.S. state of Ohio and the county seat of Montgomery County. A small part of the city extends into Greene County. The 2020 U.S. census estimate put the city population at 137,644, while Greater Da ...
, Ohio, by the Dayton Power & Light Co.; because of the thermal conductivity and very low viscosity of hydrogen gas, thus lower drag than air, this is the most common type in its field today for large generators (typically 60 MW and bigger; smaller generators are usually air-cooled
Air-cooled engines rely on the circulation of air directly over heat dissipation fins or hot areas of the engine to cool them in order to keep the engine within operating temperatures. In all combustion engines, a great percentage of the heat ge ...
).
The nickel hydrogen battery
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to r ...
was used for the first time in 1977 aboard the U.S. Navy's Navigation technology satellite-2 (NTS-2). For example, the ISS
The International Space Station (ISS) is the largest modular space station currently in low Earth orbit. It is a multinational collaborative project involving five participating space agencies: NASA (United States), Roscosmos (Russia), JAXA (J ...
, Mars Odyssey
''2001 Mars Odyssey'' is a robotic spacecraft orbiting the planet Mars. The project was developed by NASA, and contracted out to Lockheed Martin, with an expected cost for the entire mission of US$297 million. Its mission is to use spectro ...
and the Mars Global Surveyor
''Mars Global Surveyor'' (MGS) was an American robotic space probe developed by NASA's Jet Propulsion Laboratory and launched November 1996. MGS was a global mapping mission that examined the entire planet, from the ionosphere down through t ...
are equipped with nickel-hydrogen batteries. In the dark part of its orbit, the Hubble Space Telescope
The Hubble Space Telescope (often referred to as HST or Hubble) is a space telescope that was launched into low Earth orbit in 1990 and remains in operation. It was not the first space telescope, but it is one of the largest and most vers ...
is also powered by nickel-hydrogen batteries, which were finally replaced in May 2009, more than 19 years after launch and 13 years beyond their design life.
Role in quantum theory
atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, ...
ic structure. Furthermore, study of the corresponding simplicity of the hydrogen molecule and the corresponding cation brought understanding of the nature of the chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of ...
, which followed shortly after the quantum mechanical treatment of the hydrogen atom had been developed in the mid-1920s.
One of the first quantum effects to be explicitly noticed (but not understood at the time) was a Maxwell observation involving hydrogen, half a century before full quantum mechanical theory arrived. Maxwell observed that the specific heat capacity
In thermodynamics, the specific heat capacity (symbol ) of a substance is the heat capacity of a sample of the substance divided by the mass of the sample, also sometimes referred to as massic heat capacity. Informally, it is the amount of heat t ...
of unaccountably departs from that of a diatomic gas below room temperature and begins to increasingly resemble that of a monatomic gas at cryogenic temperatures. According to quantum theory, this behavior arises from the spacing of the (quantized) rotational energy levels, which are particularly wide-spaced in because of its low mass. These widely spaced levels inhibit equal partition of heat energy into rotational motion in hydrogen at low temperatures. Diatomic gases composed of heavier atoms do not have such widely spaced levels and do not exhibit the same effect.
Antihydrogen () is the antimatter
In modern physics, antimatter is defined as matter composed of the antiparticles (or "partners") of the corresponding particles in "ordinary" matter. Antimatter occurs in natural processes like cosmic ray collisions and some types of radioac ...
counterpart to hydrogen. It consists of an antiproton
The antiproton, , (pronounced ''p-bar'') is the antiparticle of the proton. Antiprotons are stable, but they are typically short-lived, since any collision with a proton will cause both particles to be annihilated in a burst of energy.
The exis ...
with a positron. Antihydrogen is the only type of antimatter atom to have been produced .
Cosmic prevalence and distribution
 Hydrogen, as atomic H, is the most abundant
Hydrogen, as atomic H, is the most abundant chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
in the universe, making up 75 percent of normal matter by mass
Mass is an intrinsic property of a body. It was traditionally believed to be related to the quantity of matter in a physical body, until the discovery of the atom and particle physics. It was found that different atoms and different eleme ...
and more than 90 percent by number of atoms. (Most of the mass of the universe, however, is not in the form of chemical-element type matter, but rather is postulated to occur as yet-undetected forms of mass such as dark matter
Dark matter is a hypothetical form of matter thought to account for approximately 85% of the matter in the universe. Dark matter is called "dark" because it does not appear to interact with the electromagnetic field, which means it does not a ...
and dark energy
In physical cosmology and astronomy, dark energy is an unknown form of energy that affects the universe on the largest scales. The first observational evidence for its existence came from measurements of supernovas, which showed that the univ ...
.) This element is found in great abundance in stars and gas giant
A gas giant is a giant planet composed mainly of hydrogen and helium. Gas giants are also called failed stars because they contain the same basic elements as a star. Jupiter and Saturn are the gas giants of the Solar System. The term "gas giant" ...
planets. Molecular cloud
A molecular cloud, sometimes called a stellar nursery (if star formation is occurring within), is a type of interstellar cloud, the density and size of which permit absorption nebulae, the formation of molecules (most commonly molecular hydroge ...
s of are associated with star formation. Hydrogen plays a vital role in powering stars through the proton-proton reaction in case of stars with very low to approximately 1 mass of the Sun and the CNO cycle
The CNO cycle (for carbon–nitrogen–oxygen; sometimes called Bethe–Weizsäcker cycle after Hans Albrecht Bethe and Carl Friedrich von Weizsäcker) is one of the two known sets of fusion reactions by which stars convert hydrogen to helium, ...
of nuclear fusion
Nuclear fusion is a reaction in which two or more atomic nuclei are combined to form one or more different atomic nuclei and subatomic particles ( neutrons or protons). The difference in mass between the reactants and products is manife ...
in case of stars more massive than our Sun
The Sun is the star at the center of the Solar System. It is a nearly perfect ball of hot plasma, heated to incandescence by nuclear fusion reactions in its core. The Sun radiates this energy mainly as light, ultraviolet, and infrared radi ...
.
States
Throughout the universe, hydrogen is mostly found in theatom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, ...
ic and plasma states, with properties quite distinct from those of molecular hydrogen. As a plasma, hydrogen's electron and proton are not bound together, resulting in very high electrical conductivity and high emissivity (producing the light from the Sun and other stars). The charged particles are highly influenced by magnetic and electric fields. For example, in the solar wind
The solar wind is a stream of charged particles released from the upper atmosphere of the Sun, called the corona. This plasma mostly consists of electrons, protons and alpha particles with kinetic energy between . The composition of the sol ...
they interact with the Earth's magnetosphere giving rise to Birkeland current
A Birkeland current (also known as field-aligned current) is a set of electrical currents that flow along geomagnetic field lines connecting the Earth's magnetosphere to the Earth's high latitude ionosphere. In the Earth's magnetosphere, the curr ...
s and the aurora
An aurora (plural: auroras or aurorae), also commonly known as the polar lights, is a natural light display in Earth's sky, predominantly seen in high-latitude regions (around the Arctic and Antarctic). Auroras display dynamic patterns of bri ...
.
Hydrogen is found in the neutral atomic state in the interstellar medium because the atoms seldom collide and combine. They are the source of the 21-cm hydrogen line at 1420 MHz that is detected in order to probe primordial hydrogen. The large amount of neutral hydrogen found in the damped Lyman-alpha system
Damped Lyman alpha systems or Damped Lyman alpha absorption systems is a term used by astronomers for concentrations of neutral hydrogen gas that are detected in the spectra of quasars – a class of distant Active Galactic Nuclei. They are defin ...
s is thought to dominate the cosmological
Cosmology () is a branch of physics and metaphysics dealing with the nature of the universe. The term ''cosmology'' was first used in English in 1656 in Thomas Blount's ''Glossographia'', and in 1731 taken up in Latin by German philosopher ...
baryon
In particle physics, a baryon is a type of composite subatomic particle which contains an odd number of valence quarks (at least 3). Baryons belong to the hadron family of particles; hadrons are composed of quarks. Baryons are also classif ...
ic density of the universe up to a redshift of ''z'' = 4.
Under ordinary conditions on Earth, elemental hydrogen exists as the diatomic gas, . Hydrogen gas is very rare in the Earth's atmosphere (1 ppm by volume) because of its light weight, which enables it to escape from the atmosphere more rapidly than heavier gases. However, hydrogen is the third most abundant element on the Earth's surface, mostly in the form of chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
s such as hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
s and water.
A molecular form called protonated molecular hydrogen () is found in the interstellar medium, where it is generated by ionization of molecular hydrogen from cosmic ray
Cosmic rays are high-energy particles or clusters of particles (primarily represented by protons or atomic nuclei) that move through space at nearly the speed of light. They originate from the Sun, from outside of the Solar System in our own ...
s. This ion has also been observed in the upper atmosphere of the planet Jupiter
Jupiter is the fifth planet from the Sun and the largest in the Solar System. It is a gas giant with a mass more than two and a half times that of all the other planets in the Solar System combined, but slightly less than one-thousandth t ...
. The ion is relatively stable in the environment of outer space due to the low temperature and density. is one of the most abundant ions in the universe, and it plays a notable role in the chemistry of the interstellar medium. Neutral triatomic hydrogen
Triatomic hydrogen or H3 is an unstable triatomic molecule containing only hydrogen. Since this molecule contains only three atoms of hydrogen it is the simplest triatomic molecule and it is relatively simple to numerically solve the quantum mechan ...
can exist only in an excited form and is unstable. By contrast, the positive hydrogen molecular ion
The dihydrogen cation or hydrogen molecular ion is a cation (positive ion) with formula . It consists of two hydrogen nuclei (protons) sharing a single electron. It is the simplest molecular ion.
The ion can be formed from the ionization of a n ...
() is a rare molecule in the universe.
Production
is produced in chemistry and biology laboratories, often as a by-product of other reactions; in industry for thehydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organ ...
of unsaturated substrates; and in nature as a means of expelling reducing equivalents in biochemical reactions.
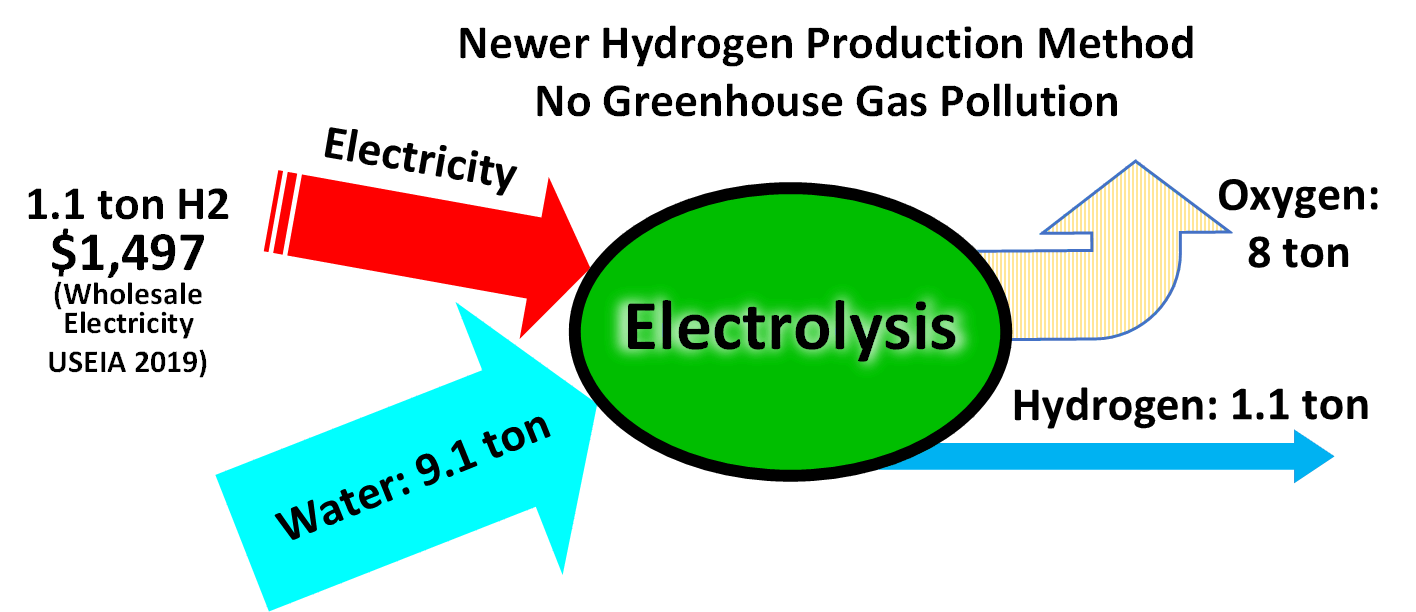
Water electrolysis
 The
The electrolysis of water
Electrolysis of water, also known as electrochemical water splitting, is the process of using electricity to decompose water into oxygen and hydrogen gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, or remi ...
is a simple method of producing hydrogen. A current is run through the water, and gaseous oxygen forms at the anode
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemonic ...
while gaseous hydrogen forms at the cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. A conventional current describes the direction in wh ...
. Typically the cathode is made from platinum or another inert metal when producing hydrogen for storage. If, however, the gas is to be burnt on site, oxygen is desirable to assist the combustion, and so both electrodes would be made from inert metals. (Iron, for instance, would oxidize, and thus decrease the amount of oxygen given off.) The theoretical maximum efficiency (electricity used vs. energetic value of hydrogen produced) is in the range 88–94%.
:
Methane pyrolysis
 Hydrogen production using natural gas methane
Hydrogen production using natural gas methane pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''py ...
is a one-step process that produces no greenhouse gases. Developing volume production using this method is the key to enabling faster carbon reduction by using hydrogen in industrial processes, fuel cell electric heavy truck transportation, and in gas turbine electric power generation. Methane pyrolysis is performed by having methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Ea ...
bubbled up through a molten metal catalyst containing dissolved nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
at . This causes the methane to break down into hydrogen gas and solid carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
, with no other byproducts.
: (ΔH° = 74 kJ/mol)
The industrial quality solid carbon may be sold as manufacturing feedstock or permanently landfilled; it is not released into the atmosphere and does not cause ground water pollution in landfill. Methane pyrolysis is in development and considered suitable for commercial bulk hydrogen production. Volume production is being evaluated in the BASF
BASF SE () is a German multinational chemical company and the largest chemical producer in the world. Its headquarters is located in Ludwigshafen, Germany.
The BASF Group comprises subsidiaries and joint ventures in more than 80 countries ...
"methane pyrolysis at scale" pilot plant. Further research continues in several laboratories, including at Karlsruhe Liquid-metal Laboratory (KALLA) and the chemical engineering laboratory at University of California – Santa Barbara
Other industrial methods
 Hydrogen is often produced by reacting water with
Hydrogen is often produced by reacting water with methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Ea ...
and carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
, which causes the removal of hydrogen from hydrocarbons at very high temperatures, with 48% of hydrogen production coming from steam reforming
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly natural gas is the feedstock. The main purpose of this technology is hydrogen product ...
. The water vapor is then reacted with the carbon monoxide produced by steam reforming to oxidize it to carbon dioxide and turn the water into hydrogen. Commercial bulk hydrogen is usually produced by the steam reforming of natural gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbo ...
with release of atmospheric greenhouse gas or with capture using CCS and climate change mitigation. Steam reforming is also known as the Bosch process and is widely used for the industrial preparation of hydrogen.
At high temperatures (1000–1400 K, 700–1100 °C or 1300–2000 °F), steam (water vapor) reacts with methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Ea ...
to yield carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
and .
:
This reaction is favored at low pressures but is nonetheless conducted at high pressures (2.0 MPa, 20 atm or 600 inHg). This is because high-pressure is the most marketable product, and pressure swing adsorption
Pressure swing adsorption (PSA) is a technique used to separate some gas species from a mixture of gases (typically air) under pressure according to the species' molecular characteristics and affinity for an adsorbent material. It operates at ne ...
(PSA) purification systems work better at higher pressures. The product mixture is known as "synthesis gas
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide, in various ratios. The gas often contains some carbon dioxide and methane. It is principly used for producing ammonia or methanol. Syngas is combustible and can be used ...
" because it is often used directly for the production of methanol and related compounds. Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
s other than methane can be used to produce synthesis gas with varying product ratios. One of the many complications to this highly optimized technology is the formation of coke or carbon:
:
Consequently, steam reforming typically employs an excess of . Additional hydrogen can be recovered from the steam by use of carbon monoxide through the water gas shift reaction
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a s ...
, especially with an iron oxide catalyst. This reaction is also a common industrial source of carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is trans ...
:
:
Other important methods for CO and production include partial oxidation of hydrocarbons:
:
and the coal reaction, which can serve as a prelude to the shift reaction above:
:
Hydrogen is sometimes produced and consumed in the same industrial process, without being separated. In the Haber process
The Haber process, also called the Haber–Bosch process, is an artificial nitrogen fixation process and is the main industrial procedure for the production of ammonia today. It is named after its inventors, the German chemists Fritz Haber and ...
for the production of ammonia, hydrogen is generated from natural gas. Electrolysis of brine to yield chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine i ...
also produces hydrogen as a co-product.
Olefin production units may produce substantial quantities of byproduct hydrogen particularly from cracking light feedstocks like ethane
Ethane ( , ) is an organic chemical compound with chemical formula . At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is isolated on an industrial scale from natural gas and as a petroc ...
or propane.
Metal-acid
Many metals react with water to produce , but the rate of hydrogen evolution depends on the metal, the pH, and the presence alloying agents. Most commonly, hydrogen evolution is induced by acids. The alkali and alkaline earth metals, aluminium, zinc, manganese, and iron react readily with aqueous acids. This reaction is the basis of the Kipp's apparatus, which once was used as a laboratory gas source: : In the absence of acid, the evolution of is slower. Because iron is widely used structural material, its anaerobic corrosion is of technological significance: : Many metals, such asaluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
, are slow to react with water because they form passivated coatings of oxides. An alloy of aluminium and gallium, however, does react with water. At high pH, aluminium can produce :
:
Some metal-containing compounds react with acids to evolve . Under anaerobic conditions, ferrous hydroxide
Iron(II) hydroxide or ferrous hydroxide is an inorganic compound with the formula Fe(OH)2. It is produced when iron(II) salts, from a compound such as iron(II) sulfate, are treated with hydroxide ions. Iron(II) hydroxide is a white solid, but eve ...
() can be oxidized by the protons of water to form magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula Fe2+Fe3+2O4. It is one of the oxides of iron, and is ferrimagnetic; it is attracted to a magnet and can be magnetized to become a permanent magnet itself. With th ...
and . This process is described by the Schikorr reaction:
:
This process occurs during the anaerobic corrosion of iron
Iron () is a chemical element with Symbol (chemistry), symbol Fe (from la, Wikt:ferrum, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 element, group 8 of the periodic table. It is, Abundanc ...
and steel in oxygen-free groundwater
Groundwater is the water present beneath Earth's surface in rock and soil pore spaces and in the fractures of rock formations. About 30 percent of all readily available freshwater in the world is groundwater. A unit of rock or an unconsolidated ...
and in reducing soil
Soil, also commonly referred to as earth or dirt
Dirt is an unclean matter, especially when in contact with a person's clothes, skin, or possessions. In such cases, they are said to become dirty.
Common types of dirt include:
* Debri ...
s below the water table
The water table is the upper surface of the zone of saturation. The zone of saturation is where the pores and fractures of the ground are saturated with water. It can also be simply explained as the depth below which the ground is saturated.
T ...
.
Thermochemical
More than 200 thermochemical cycles can be used forwater splitting
Water splitting is the chemical reaction in which water is broken down into oxygen and hydrogen:
:2 H2O → 2 H2 + O2
Efficient and economical water splitting would be a technological breakthrough that could underpin a hydrogen economy, base ...
. Many of these cycles such as the iron oxide cycle
For chemical reactions, the iron oxide cycle (Fe3O4/FeO) is the original two-step thermochemical cycle proposed for use for hydrogen production.
It is based on the reduction and subsequent oxidation of iron ions, particularly the reduction and oxid ...
, cerium(IV) oxide–cerium(III) oxide cycle
The cerium(IV) oxide–cerium(III) oxide cycle or CeO2/Ce2O3 cycle is a two-step thermochemical process that employs cerium(IV) oxide and cerium(III) oxide for hydrogen production. The cerium-based cycle allows the separation of H2 and O2 in two s ...
, zinc zinc-oxide cycle
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic ta ...
, sulfur-iodine cycle, copper-chlorine cycle and hybrid sulfur cycle
The hybrid sulfur cycle (HyS) is a two-step water-splitting process intended to be used for hydrogen production. Based on sulfur oxidation and reduction, it is classified as a hybrid thermochemical cycle because it uses an electrochemical (inste ...
have been evaluated for their commercial potential to produce hydrogen and oxygen from water and heat without using electricity. A number of laboratories (including in France
France (), officially the French Republic ( ), is a country primarily located in Western Europe. It also comprises of overseas regions and territories in the Americas and the Atlantic, Pacific and Indian Oceans. Its metropolitan area ...
, Germany
Germany,, officially the Federal Republic of Germany, is a country in Central Europe. It is the second most populous country in Europe after Russia, and the most populous member state of the European Union. Germany is situated betwe ...
, Greece
Greece,, or , romanized: ', officially the Hellenic Republic, is a country in Southeast Europe. It is situated on the southern tip of the Balkans, and is located at the crossroads of Europe, Asia, and Africa. Greece shares land borders ...
, Japan, and the United States
The United States of America (U.S.A. or USA), commonly known as the United States (U.S. or US) or America, is a country primarily located in North America. It consists of 50 states, a federal district, five major unincorporated territori ...
) are developing thermochemical methods to produce hydrogen from solar energy and water.
Serpentinization reaction
In deep geological conditions prevailing far away from the Earth's atmosphere, hydrogen () is produced during the process ofserpentinization
Serpentinization is a hydration and metamorphic transformation of ferromagnesian minerals, such as olivine and pyroxene, in mafic and ultramafic rock to produce serpentinite. Minerals formed by serpentinization include the serpentine group mine ...
. In this process, water protons () are reduced by ferrous () ions provided by fayalite
Fayalite (, commonly abbreviated to Fa) is the iron-rich end-member of the olivine solid-solution series. In common with all minerals in the olivine group, fayalite crystallizes in the orthorhombic system (space group ''Pbnm'') with cell parame ...
(). The reaction forms magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula Fe2+Fe3+2O4. It is one of the oxides of iron, and is ferrimagnetic; it is attracted to a magnet and can be magnetized to become a permanent magnet itself. With th ...
(), quartz
Quartz is a hard, crystalline mineral composed of silica ( silicon dioxide). The atoms are linked in a continuous framework of SiO4 silicon-oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall chemical ...
(), and hydrogen ():
:
:''fayalite + water → magnetite + quartz + hydrogen''
This reaction closely resembles the Schikorr reaction observed in anaerobic oxidation of ferrous hydroxide
Iron(II) hydroxide or ferrous hydroxide is an inorganic compound with the formula Fe(OH)2. It is produced when iron(II) salts, from a compound such as iron(II) sulfate, are treated with hydroxide ions. Iron(II) hydroxide is a white solid, but eve ...
in contact with water.
Applications
Petrochemical industry
Large quantities of are used in the "upgrading" of fossil fuels. Key consumers of includehydrodealkylation
Hydrodealkylation is a chemical reaction that often involves reacting an aromatic hydrocarbon, such as toluene, in the presence of hydrogen gas to form a simpler aromatic hydrocarbon devoid of functional groups. An example is the conversion of 1,2, ...
, hydrodesulfurization, and hydrocracking. Many of these reactions can be classified as hydrogenolysis
Hydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes lysis (breakdown) by hydrogen.Ralph Connor, Homer Adkins. Hydrogenolysis Of Oxygenated Organic Compounds. J. Am. Chem. Soc. ...
, i.e., the cleavage of bonds to carbon. Illustrative is the separation of sulfur from liquid fossil fuels:
:
Hydrogenation
Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organ ...
, the addition of to various substrates is conducted on a large scale. The hydrogenation of to produce ammonia by the Haber–Bosch process
The Haber process, also called the Haber–Bosch process, is an artificial nitrogen fixation process and is the main industrial procedure for the production of ammonia today. It is named after its inventors, the German chemists Fritz Haber and C ...
consumes a few percent of the energy budget in the entire industry. The resulting ammonia is used to supply the majority of the protein consumed by humans. Hydrogenation is used to convert unsaturated fat
An unsaturated fat is a fat or fatty acid in which there is at least one double bond within the fatty acid chain. A fatty acid chain is monounsaturated if it contains one double bond, and polyunsaturated if it contains more than one double bond.
...
s and oils
An oil is any nonpolar chemical substance that is composed primarily of hydrocarbons and is hydrophobic (does not mix with water) & lipophilic (mixes with other oils). Oils are usually flammable and surface active. Most oils are unsaturate ...
to saturated fats and oils. The major application is the production of margarine. Methanol is produced by hydrogenation of carbon dioxide. It is similarly the source of hydrogen in the manufacture of hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
. is also used as a reducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are commonly reducing agents include the Earth me ...
for the conversion of some ore
Ore is natural rock or sediment that contains one or more valuable minerals, typically containing metals, that can be mined, treated and sold at a profit.Encyclopædia Britannica. "Ore". Encyclopædia Britannica Online. Retrieved 7 Apr ...
s to the metals.
Coolant
Hydrogen is commonly used in power stations as a coolant in generators due to a number of favorable properties that are a direct result of its light diatomic molecules. These include lowdensity
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematical ...
, low viscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the inte ...
, and the highest specific heat
In thermodynamics, the specific heat capacity (symbol ) of a substance is the heat capacity of a sample of the substance divided by the mass of the sample, also sometimes referred to as massic heat capacity. Informally, it is the amount of heat t ...
and thermal conductivity
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by k, \lambda, or \kappa.
Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal ...
of all gases.
Energy carrier
Elemental hydrogen has been widely discussed in the context of energy, as a possible future carrier of energy on an economy-wide scale. Hydrogen is amethane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Ea ...
, the main component of natural gas. Producing one tonne of hydrogen through this process emits 6.6–9.3 tonnes of carbon dioxide. While carbon capture and storage
Carbon capture and storage (CCS) or carbon capture and sequestration is the process of capturing carbon dioxide (CO2) before it enters the atmosphere, transporting it, and storing it (carbon sequestration) for centuries or millennia. Usually th ...
can remove a large fraction of these emissions, the overall carbon footprint of hydrogen from natural gas is difficult to assess , in part because of emissions created in the production of the natural gas itself.
Electricity can be used to split water molecules, producing sustainable hydrogen provided the electricity was generated sustainably. However, this electrolysis process is currently more expensive than creating hydrogen from methane and the efficiency of energy conversion is inherently low. Hydrogen can be produced when there is a surplus of variable renewable electricity, then stored and used to generate heat or to re-generate electricity. It can be further transformed into synthetic fuel
Synthetic fuel or synfuel is a liquid fuel, or sometimes gaseous fuel, obtained from syngas, a mixture of carbon monoxide and hydrogen, in which the syngas was derived from gasification of solid feedstocks such as coal or biomass or by refo ...
s such as ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous wa ...
and methanol.
Innovation in hydrogen electrolysers could make large-scale production of hydrogen from electricity more cost-competitive. There is potential for hydrogen to play a significant role in decarbonising energy systems because in certain sectors, replacing fossil fuels with direct use of electricity would be very difficult. Hydrogen fuel can produce the intense heat required for industrial production of steel, cement, glass, and chemicals. For steelmaking, hydrogen can function as a clean energy carrier and simultaneously as a low-carbon catalyst replacing coal-derived coke. Hydrogen used in transportation would burn relatively cleanly, with some emissions, but without carbon emissions. Disadvantages of hydrogen as an energy carrier include high costs of storage and distribution due to hydrogen's explosivity, its large volume compared to other fuels, and its tendency to make pipes brittle. The infrastructure costs associated with full conversion to a hydrogen economy would be substantial.
Semiconductor industry
Hydrogen is employed to saturate broken ("dangling") bonds ofamorphous silicon
Amorphous silicon (a-Si) is the non-crystalline form of silicon used for solar cells and thin-film transistors in LCDs.
Used as semiconductor material for a-Si solar cells, or thin-film silicon solar cells, it is deposited in thin films ont ...
and amorphous carbon Amorphous carbon is free, reactive carbon that has no crystalline structure. Amorphous carbon materials may be stabilized by terminating dangling-π bonds with hydrogen. As with other amorphous solids, some short-range order can be observed. Amor ...
that helps stabilizing material properties. It is also a potential electron donor
In chemistry, an electron donor is a chemical entity that donates electrons to another compound. It is a reducing agent that, by virtue of its donating electrons, is itself oxidized in the process.
Typical reducing agents undergo permanent chemi ...
in various oxide materials, including ZnO, , CdO, MgO
Magnesium oxide ( Mg O), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions ...
, , , , , , , , , , , , and .
Aerospace
Liquid hydrogen
Liquid hydrogen (LH2 or LH2) is the liquid state of the element hydrogen. Hydrogen is found naturally in the molecular H2 form.
To exist as a liquid, H2 must be cooled below its critical point of 33 K. However, for it to be in a fully l ...
and liquid oxygen
Liquid oxygen—abbreviated LOx, LOX or Lox in the aerospace, submarine and gas industries—is the liquid form of molecular oxygen. It was used as the oxidizer in the first liquid-fueled rocket invented in 1926 by Robert H. Goddard, an app ...
together serve as cryogenic fuel
Cryogenic fuels are fuels that require storage at extremely low temperatures in order to maintain them in a liquid state. These fuels are used in machinery that operates in space (e.g. rockets and satellites) where ordinary fuel cannot be used, d ...
in liquid-propellant rockets, as in the Space Shuttle main engines
The Aerojet Rocketdyne RS-25, also known as the Space Shuttle Main Engine (SSME), is a liquid-fuel cryogenic rocket engine that was used on NASA's Space Shuttle and is currently used on the Space Launch System (SLS).
Designed and manufacture ...
.
Niche and evolving uses
*Shielding gas: Hydrogen is used as ashielding gas
Shielding gases are inert or semi-inert gases that are commonly used in several welding processes, most notably gas metal arc welding and gas tungsten arc welding (GMAW and GTAW, more popularly known as MIG (Metal Inert Gas) and TIG (Tungsten Iner ...
in welding
Welding is a fabrication process that joins materials, usually metals or thermoplastics, by using high heat to melt the parts together and allowing them to cool, causing fusion. Welding is distinct from lower temperature techniques such as bra ...
methods such as atomic hydrogen welding.
*Cryogenic research: Liquid is used in cryogenic research, including superconductivity studies.
*Buoyant lifting: Because is lighter than air, having only 7% of the density of air, it was once widely used as a lifting gas
A lifting gas or lighter-than-air gas is a gas that has a density lower than normal atmospheric gases and rises above them as a result. It is required for aerostats to create buoyancy, particularly in lighter-than-air aircraft, which include ballo ...
in balloons and airship
An airship or dirigible balloon is a type of aerostat or lighter-than-air aircraft that can navigate through the air under its own power. Aerostats gain their lift from a lifting gas that is less dense than the surrounding air.
In early ...
s.
*Leak detection: Pure or mixed with nitrogen (sometimes called forming gas
Forming gas is a mixture of hydrogen (mole fraction varies) and nitrogen. It is sometimes called a "dissociated ammonia atmosphere" due to the reaction which generates it:
:2 NH3 → 3 H2 + N2
It can also be manufactured by thermal cra ...
), hydrogen is a tracer gas
A tracer-gas leak testing method is a nondestructive testing method that detects gas leaks. A variety of methods with different sensitivities exist. Tracer-gas leak testing is used in the petrochemical industry, the automotive industry, and in the ...
for detection {{Unreferenced, date=March 2018
In general, detection is the action of accessing information without specific cooperation from with the sender.
In the history of radio communications, the term " detector" was first used for a device that detected ...
of minute leaks. Applications can be found in the automotive, chemical, power generation, aerospace, and telecommunications industries. Hydrogen is an authorized food additive (E 949) that allows food package leak testing, as well as having anti-oxidizing properties.
*Neutron moderation: Deuterium
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two stable isotopes of hydrogen (the other being protium, or hydrogen-1). The nucleus of a deuterium atom, called a deuteron, contains one proton and one ...
(hydrogen-2) is used in nuclear fission applications as a moderator to slow neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons beh ...
s.
*Nuclear fusion fuel: Deuterium is used in nuclear fusion
Nuclear fusion is a reaction in which two or more atomic nuclei are combined to form one or more different atomic nuclei and subatomic particles ( neutrons or protons). The difference in mass between the reactants and products is manife ...
reactions.
*Isotopic labeling: Deuterium compounds have applications in chemistry and biology in studies of isotope effects on reaction rates.
*Rocket propellant: NASA
The National Aeronautics and Space Administration (NASA ) is an independent agencies of the United States government, independent agency of the US federal government responsible for the civil List of government space agencies, space program ...
has investigated the use of rocket propellant made from atomic hydrogen, boron or carbon that is frozen into solid molecular hydrogen particles that are suspended in liquid helium. Upon warming, the mixture vaporizes to allow the atomic species to recombine, heating the mixture to high temperature.
*Tritium uses: Tritium
Tritium ( or , ) or hydrogen-3 (symbol T or H) is a rare and radioactive isotope of hydrogen with half-life about 12 years. The nucleus of tritium (t, sometimes called a ''triton'') contains one proton and two neutrons, whereas the nucleus of ...
(hydrogen-3), produced in nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat fr ...
s, is used in the production of hydrogen bombs, as an isotopic label in the biosciences, and as a source of beta radiation
A beta particle, also called beta ray or beta radiation (symbol β), is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus during the process of beta decay. There are two forms of beta decay, β� ...
in radioluminescent paint
Luminous paint or luminescent paint is paint that exhibits luminescence. In other words, it gives off visible light through fluorescence, phosphorescence, or radioluminescence. There are three types of luminous paints: fluorescent paint, phosp ...
for instrument dials and emergency signage.
Biological reactions
is a product of some types ofanaerobic metabolism
Anaerobic respiration is respiration using electron acceptors other than molecular oxygen (O2). Although oxygen is not the final electron acceptor, the process still uses a respiratory electron transport chain.
In aerobic organisms undergoing r ...
and is produced by several microorganism
A microorganism, or microbe,, ''mikros'', "small") and ''organism'' from the el, ὀργανισμός, ''organismós'', "organism"). It is usually written as a single word but is sometimes hyphenated (''micro-organism''), especially in olde ...
s, usually via reactions catalyzed
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
by iron
Iron () is a chemical element with Symbol (chemistry), symbol Fe (from la, Wikt:ferrum, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 element, group 8 of the periodic table. It is, Abundanc ...
- or nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
-containing enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
s called hydrogenase A hydrogenase is an enzyme that catalyses the reversible oxidation of molecular hydrogen (H2), as shown below:
Hydrogen uptake () is coupled to the reduction of electron acceptors such as oxygen, nitrate, sulfate, carbon dioxide (), and fumarat ...
s. These enzymes catalyze the reversible redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
reaction between and its component two protons and two electrons. Creation of hydrogen gas occurs in the transfer of reducing equivalents produced during pyruvate fermentation to water. The natural cycle of hydrogen production and consumption by organisms is called the hydrogen cycle. Hydrogen is the most abundant element in the human body in terms of numbers of atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, ...
s of the element but, it is the 3rd most abundant element by mass, because hydrogen is so light. occurs in the breath of humans due to the metabolic activity of hydrogenase-containing microorganisms in the large intestine
The large intestine, also known as the large bowel, is the last part of the gastrointestinal tract and of the digestive system in tetrapods. Water is absorbed here and the remaining waste material is stored in the rectum as feces before bein ...
. The concentration in fasted people at rest is typically less than 5 parts per million
In science and engineering, the parts-per notation is a set of pseudo-units to describe small values of miscellaneous dimensionless quantities, e.g. mole fraction or mass fraction. Since these fractions are quantity-per-quantity measures, th ...
(ppm) but can be 50 ppm when people with intestinal disorders consume molecules they cannot absorb during diagnostic hydrogen breath test
A hydrogen breath test (or HBT) is used as a diagnostic tool for small intestine bacterial overgrowth and carbohydrate malabsorption, such as lactose, fructose, and sorbitol malabsorption.
The test is simple, non-invasive, and is performed after ...
s.
Hydrogen gas is produced by some bacteria and algae and is a natural component of flatus
Flatulence, in humans, is the expulsion of gas from the intestines via the anus, commonly referred to as farting. "Flatus" is the medical word for gas generated in the stomach or bowels. A proportion of intestinal gas may be swallowed environm ...
, as is methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Ea ...
, itself a hydrogen source of increasing importance.
Water splitting
Water splitting is the chemical reaction in which water is broken down into oxygen and hydrogen:
:2 H2O → 2 H2 + O2
Efficient and economical water splitting would be a technological breakthrough that could underpin a hydrogen economy, base ...
, in which water is decomposed into its component protons, electrons, and oxygen, occurs in the light reactions in all photosynthetic organisms. Some such organisms, including the alga ''Chlamydomonas reinhardtii
''Chlamydomonas reinhardtii'' is a single-cell green alga about 10 micrometres in diameter that swims with two flagella. It has a cell wall made of hydroxyproline-rich glycoproteins, a large cup-shaped chloroplast, a large pyrenoid, and an eye ...
'' and cyanobacteria, have evolved a second step in the dark reactions in which protons and electrons are reduced to form gas by specialized hydrogenases in the chloroplast. Efforts have been undertaken to genetically modify cyanobacterial hydrogenases to efficiently synthesize gas even in the presence of oxygen. Efforts have also been undertaken with genetically modified alga in a bioreactor.
Safety and precautions
Hydrogen poses a number of hazards to human safety, from potentialdetonation
Detonation () is a type of combustion involving a supersonic exothermic front accelerating through a medium that eventually drives a shock front propagating directly in front of it. Detonations propagate supersonically through shock waves with s ...
s and fires when mixed with air to being an asphyxia
Asphyxia or asphyxiation is a condition of deficient supply of oxygen to the body which arises from abnormal breathing. Asphyxia causes generalized hypoxia, which affects primarily the tissues and organs. There are many circumstances that ca ...
nt in its pure, oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
-free form. In addition, liquid hydrogen is a cryogen
In physics, cryogenics is the production and behaviour of materials at very low temperatures.
The 13th IIR International Congress of Refrigeration (held in Washington DC in 1971) endorsed a universal definition of “cryogenics” and “cr ...
and presents dangers (such as frostbite
Frostbite is a skin injury that occurs when exposed to extreme low temperatures, causing the freezing of the skin or other tissues, commonly affecting the fingers, toes, nose, ears, cheeks and chin areas. Most often, frostbite occurs in the ha ...
) associated with very cold liquids. Hydrogen dissolves in many metals and in addition to leaking out, may have adverse effects on them, such as hydrogen embrittlement
Hydrogen embrittlement (HE), also known as hydrogen-assisted cracking or hydrogen-induced cracking (HIC), is a reduction in the ductility of a metal due to absorbed hydrogen. Hydrogen atoms are small and can permeate solid metals. Once absorbed ...
, leading to cracks and explosions. Hydrogen gas leaking into external air may spontaneously ignite. Moreover, hydrogen fire, while being extremely hot, is almost invisible, and thus can lead to accidental burns.
Even interpreting the hydrogen data (including safety data) is confounded by a number of phenomena. Many physical and chemical properties of hydrogen depend on the parahydrogen/orthohydrogen ratio (it often takes days or weeks at a given temperature to reach the equilibrium ratio, for which the data is usually given). Hydrogen detonation parameters, such as critical detonation pressure and temperature, strongly depend on the container geometry.
See also
* * * * * * * (for hydrogen) * *Notes
References
Further reading
* * * * * * *Hydrogen safety
Hydrogen safety covers the safe production, handling and use of hydrogen, particularly hydrogen gas fuel and liquid hydrogen.
Hydrogen possesses the NFPA 704's highest rating of 4 on the flammability scale because it is flammable when mixed even i ...
covers the safe production, handling and use
External links
Basic Hydrogen Calculations of Quantum Mechanics
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics i ...
'' (University of Nottingham)
{{Authority control Chemical elements Reactive nonmetals Diatomic nonmetals Nuclear fusion fuels Airship technology Reducing agents Refrigerants Gaseous signaling molecules E-number additives