Hemoglobin Peroxidase on:
[Wikipedia]
[Google]
[Amazon]
Hemoglobin (haemoglobin
 In 1825, Johann Friedrich Engelhart discovered that the ratio of iron to protein is identical in the hemoglobins of several species. From the known atomic mass of iron he calculated the molecular mass of hemoglobin to ''n'' × 16000 (''n'' = number of iron atoms per hemoglobin, now known to be 4), the first determination of a protein's molecular mass. This "hasty conclusion" drew a lot of ridicule at the time from scientists who could not believe that any molecule could be that big. Gilbert Smithson Adair confirmed Engelhart's results in 1925 by measuring the osmotic pressure of hemoglobin solutions.
Although blood had been known to carry oxygen since at least 1794, the oxygen-carrying property of hemoglobin was described by Hünefeld in 1840. In 1851, German physiologist
In 1825, Johann Friedrich Engelhart discovered that the ratio of iron to protein is identical in the hemoglobins of several species. From the known atomic mass of iron he calculated the molecular mass of hemoglobin to ''n'' × 16000 (''n'' = number of iron atoms per hemoglobin, now known to be 4), the first determination of a protein's molecular mass. This "hasty conclusion" drew a lot of ridicule at the time from scientists who could not believe that any molecule could be that big. Gilbert Smithson Adair confirmed Engelhart's results in 1925 by measuring the osmotic pressure of hemoglobin solutions.
Although blood had been known to carry oxygen since at least 1794, the oxygen-carrying property of hemoglobin was described by Hünefeld in 1840. In 1851, German physiologist
 Variations in hemoglobin amino acid sequences, as with other proteins, may be adaptive. For example, hemoglobin has been found to adapt in different ways to high altitudes. Organisms living at high elevations experience lower partial pressures of oxygen compared to those at sea level. This presents a challenge to the organisms that inhabit such environments because hemoglobin, which normally binds oxygen at high partial pressures of oxygen, must be able to bind oxygen when it is present at a lower pressure. Different organisms have adapted to such a challenge. For example, recent studies have suggested genetic variants in deer mice that help explain how deer mice that live in the mountains are able to survive in the thin air that accompanies high altitudes. A researcher from the University of Nebraska-Lincoln found mutations in four different genes that can account for differences between deer mice that live in lowland prairies versus the mountains. After examining wild mice captured from both highlands and lowlands, it was found that: the genes of the two breeds are "virtually identical—except for those that govern the oxygen-carrying capacity of their hemoglobin". "The genetic difference enables highland mice to make more efficient use of their oxygen", since less is available at higher altitudes, such as those in the mountains. Mammoth hemoglobin featured mutations that allowed for oxygen delivery at lower temperatures, thus enabling mammoths to migrate to higher latitudes during the Pleistocene. This was also found in hummingbirds that inhabit the Andes. Hummingbirds already expend a lot of energy and thus have high oxygen demands and yet Andean hummingbirds have been found to thrive in high altitudes. Non-synonymous mutations in the hemoglobin gene of multiple species living at high elevations (''Oreotrochilus, A. castelnaudii, C. violifer, P. gigas,'' and ''A. viridicuada'') have caused the protein to have less of an affinity for inositol hexaphosphate (IHP), a molecule found in birds that has a similar role as 2,3-BPG in humans; this results in the ability to bind oxygen in lower partial pressures.
Birds' unique circulatory lungs also promote efficient use of oxygen at low partial pressures of O2. These two adaptations reinforce each other and account for birds' remarkable high-altitude performance.
Hemoglobin adaptation extends to humans, as well. There is a higher offspring survival rate among Tibetan women with high oxygen saturation genotypes residing at 4,000 m. Natural selection seems to be the main force working on this gene because the mortality rate of offspring is significantly lower for women with higher hemoglobin-oxygen affinity when compared to the mortality rate of offspring from women with low hemoglobin-oxygen affinity. While the exact genotype and mechanism by which this occurs is not yet clear, selection is acting on these women's ability to bind oxygen in low partial pressures, which overall allows them to better sustain crucial metabolic processes.
Variations in hemoglobin amino acid sequences, as with other proteins, may be adaptive. For example, hemoglobin has been found to adapt in different ways to high altitudes. Organisms living at high elevations experience lower partial pressures of oxygen compared to those at sea level. This presents a challenge to the organisms that inhabit such environments because hemoglobin, which normally binds oxygen at high partial pressures of oxygen, must be able to bind oxygen when it is present at a lower pressure. Different organisms have adapted to such a challenge. For example, recent studies have suggested genetic variants in deer mice that help explain how deer mice that live in the mountains are able to survive in the thin air that accompanies high altitudes. A researcher from the University of Nebraska-Lincoln found mutations in four different genes that can account for differences between deer mice that live in lowland prairies versus the mountains. After examining wild mice captured from both highlands and lowlands, it was found that: the genes of the two breeds are "virtually identical—except for those that govern the oxygen-carrying capacity of their hemoglobin". "The genetic difference enables highland mice to make more efficient use of their oxygen", since less is available at higher altitudes, such as those in the mountains. Mammoth hemoglobin featured mutations that allowed for oxygen delivery at lower temperatures, thus enabling mammoths to migrate to higher latitudes during the Pleistocene. This was also found in hummingbirds that inhabit the Andes. Hummingbirds already expend a lot of energy and thus have high oxygen demands and yet Andean hummingbirds have been found to thrive in high altitudes. Non-synonymous mutations in the hemoglobin gene of multiple species living at high elevations (''Oreotrochilus, A. castelnaudii, C. violifer, P. gigas,'' and ''A. viridicuada'') have caused the protein to have less of an affinity for inositol hexaphosphate (IHP), a molecule found in birds that has a similar role as 2,3-BPG in humans; this results in the ability to bind oxygen in lower partial pressures.
Birds' unique circulatory lungs also promote efficient use of oxygen at low partial pressures of O2. These two adaptations reinforce each other and account for birds' remarkable high-altitude performance.
Hemoglobin adaptation extends to humans, as well. There is a higher offspring survival rate among Tibetan women with high oxygen saturation genotypes residing at 4,000 m. Natural selection seems to be the main force working on this gene because the mortality rate of offspring is significantly lower for women with higher hemoglobin-oxygen affinity when compared to the mortality rate of offspring from women with low hemoglobin-oxygen affinity. While the exact genotype and mechanism by which this occurs is not yet clear, selection is acting on these women's ability to bind oxygen in low partial pressures, which overall allows them to better sustain crucial metabolic processes.
MedicineNet. Web. 12 Oct. 2009. The four polypeptide chains are bound to each other by salt bridges,
 Variant forms that cause disease:
*
Variant forms that cause disease:
*
National Anemia Action Council
a
anemia.org
at ttps://www.life-of-science.net www.life-of-science.net
Animation of hemoglobin: from deoxy to oxy form
a
vimeo.com
{{Authority control Hemoglobins Equilibrium chemistry Respiratory physiology
BrE
British English (BrE, en-GB, or BE) is, according to Lexico, Oxford Dictionaries, "English language, English as used in Great Britain, as distinct from that used elsewhere". More narrowly, it can refer specifically to the English language in ...
) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocytes) of almost all vertebrates (the exception being the fish family Channichthyidae) as well as the tissues of some invertebrates. Hemoglobin in blood carries oxygen from the respiratory organs (''e.g.'' lung
The lungs are the primary organs of the respiratory system in humans and most other animals, including some snails and a small number of fish. In mammals and most other vertebrates, two lungs are located near the backbone on either side of t ...
s or gills) to the rest of the body (''i.e.'' tissues). There it releases the oxygen to permit aerobic respiration
Cellular respiration is the process by which biological fuels are oxidised in the presence of an inorganic electron acceptor such as oxygen to produce large amounts of energy, to drive the bulk production of ATP. Cellular respiration may be des ...
to provide energy to power functions of an organism in the process called metabolism. A healthy individual human has 12to 20grams of hemoglobin in every 100mL of blood.
In mammal
Mammals () are a group of vertebrate animals constituting the class Mammalia (), characterized by the presence of mammary glands which in females produce milk for feeding (nursing) their young, a neocortex (a region of the brain), fur or ...
s, the chromoprotein A chromoprotein is a conjugated protein that contains a pigmented prosthetic group (or cofactor). A common example is haemoglobin, which contains a heme cofactor, which is the iron-containing molecule that makes oxygenated blood appear red. Other e ...
makes up about 96% of the red blood cells' dry content (by weight), and around 35% of the total content (including water). Hemoglobin has an oxygen-binding capacity of 1.34mL O2 per gram, which increases the total blood oxygen capacity seventy-fold compared to dissolved oxygen in blood. The mammalian hemoglobin molecule can bind (carry) up to four oxygen molecules.
Hemoglobin is involved in the transport of other gases: It carries some of the body's respiratory carbon dioxide (about 20–25% of the total) as carbaminohemoglobin, in which CO2 is bound to the heme protein. The molecule also carries the important regulatory molecule nitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its che ...
bound to a thiol group in the globin protein, releasing it at the same time as oxygen.
Hemoglobin is also found outside red blood cells and their progenitor lines. Other cells that contain hemoglobin include the A9 dopaminergic neurons in the substantia nigra
The substantia nigra (SN) is a basal ganglia structure located in the midbrain that plays an important role in reward and movement. ''Substantia nigra'' is Latin for "black substance", reflecting the fact that parts of the substantia nigra app ...
, macrophage
Macrophages (abbreviated as M φ, MΦ or MP) ( el, large eaters, from Greek ''μακρός'' (') = large, ''φαγεῖν'' (') = to eat) are a type of white blood cell of the immune system that engulfs and digests pathogens, such as cancer cel ...
s, alveolar cells, lungs, retinal pigment epithelium, hepatocytes, mesangial cells in the kidney, endometrial cells, cervical cells and vaginal epithelial cells. In these tissues, hemoglobin has a non-oxygen-carrying function as an antioxidant
Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. This can lead to polymerization and other chain reactions. They are frequently added to industrial products, such as fuels and lubricant ...
and a regulator of iron metabolism. Excessive glucose in one's blood can attach to hemoglobin and raise the level of hemoglobin A1c.
Hemoglobin and hemoglobin-like molecules are also found in many invertebrates, fungi, and plants. In these organisms, hemoglobins may carry oxygen, or they may act to transport and regulate other small molecules and ions such as carbon dioxide, nitric oxide, hydrogen sulfide and sulfide. A variant of the molecule, called leghemoglobin, is used to scavenge oxygen away from anaerobic systems, such as the nitrogen-fixing nodules of leguminous plants, lest the oxygen poison (deactivate) the system.
Hemoglobinemia is a medical condition in which there is an excess of hemoglobin in the blood plasma. This is an effect of intravascular hemolysis, in which hemoglobin separates from red blood cells, a form of anemia.
Research history
 In 1825, Johann Friedrich Engelhart discovered that the ratio of iron to protein is identical in the hemoglobins of several species. From the known atomic mass of iron he calculated the molecular mass of hemoglobin to ''n'' × 16000 (''n'' = number of iron atoms per hemoglobin, now known to be 4), the first determination of a protein's molecular mass. This "hasty conclusion" drew a lot of ridicule at the time from scientists who could not believe that any molecule could be that big. Gilbert Smithson Adair confirmed Engelhart's results in 1925 by measuring the osmotic pressure of hemoglobin solutions.
Although blood had been known to carry oxygen since at least 1794, the oxygen-carrying property of hemoglobin was described by Hünefeld in 1840. In 1851, German physiologist
In 1825, Johann Friedrich Engelhart discovered that the ratio of iron to protein is identical in the hemoglobins of several species. From the known atomic mass of iron he calculated the molecular mass of hemoglobin to ''n'' × 16000 (''n'' = number of iron atoms per hemoglobin, now known to be 4), the first determination of a protein's molecular mass. This "hasty conclusion" drew a lot of ridicule at the time from scientists who could not believe that any molecule could be that big. Gilbert Smithson Adair confirmed Engelhart's results in 1925 by measuring the osmotic pressure of hemoglobin solutions.
Although blood had been known to carry oxygen since at least 1794, the oxygen-carrying property of hemoglobin was described by Hünefeld in 1840. In 1851, German physiologist Otto Funke
Otto Funke (October 27, 1828 – August 17, 1879) was a German physiologist born in Chemnitz.
He studied in Leipzig and Heidelberg, and in 1852, he became a lecturer of physiology at the University of Leipzig. In 1853, he became an associate pr ...
published a series of articles in which he described growing hemoglobin crystals by successively diluting red blood cells with a solvent such as pure water, alcohol or ether, followed by slow evaporation of the solvent from the resulting protein solution. Hemoglobin's reversible oxygenation was described a few years later by Felix Hoppe-Seyler.
With the development of X-ray crystallography, it became possible to sequence protein structures. In 1959, Max Perutz determined the molecular structure of hemoglobin. For this work he shared the 1962 Nobel Prize in Chemistry with John Kendrew, who sequenced the globular protein myoglobin
Myoglobin (symbol Mb or MB) is an iron- and oxygen-binding protein found in the cardiac and skeletal muscle tissue of vertebrates in general and in almost all mammals. Myoglobin is distantly related to hemoglobin. Compared to hemoglobin, myoglobi ...
.
The role of hemoglobin in the blood was elucidated by French physiologist
Physiology (; ) is the scientific study of functions and mechanisms in a living system. As a sub-discipline of biology, physiology focuses on how organisms, organ systems, individual organs, cells, and biomolecules carry out the chemical a ...
Claude Bernard.
The name ''hemoglobin'' is derived from the words '' heme'' and '' globin'', reflecting the fact that each subunit
Subunit may refer to:
*Subunit HIV vaccine, a class of HIV vaccine
*Protein subunit, a protein molecule that assembles with other protein molecules
*Monomer, a molecule that may bind chemically to other molecules to form a polymer
*Sub-subunit, a ...
of hemoglobin is a globular protein with an embedded heme group. Each heme group contains one iron atom, that can bind one oxygen molecule through ion-induced dipole forces. The most common type of hemoglobin in mammals contains four such subunits.
Genetics
Hemoglobin consists of protein subunits (the globin molecules), and these proteins, in turn, are folded chains of a large number of different amino acids called polypeptides. The amino acid sequence of any polypeptide created by a cell is in turn determined by the stretches of DNA called genes. In all proteins, it is the amino acid sequence that determines the protein's chemical properties and function. There is more than one hemoglobin gene: in humans, hemoglobin A (the main form of hemoglobin present in adults) is coded for by the genes, '' HBA1'', '' HBA2'', and ''HBB
Hemoglobin subunit beta (beta globin, β-globin, haemoglobin beta, hemoglobin beta) is a globin protein, coded for by the ''HBB'' gene, which along with alpha globin ( HBA), makes up the most common form of haemoglobin in adult humans, hemoglobi ...
''. The hemoglobin subunit alpha 1 and alpha 2 are coded by the genes ''HBA1'' and ''HBA2'', respectively, which are both on chromosome 16 and are close to each other. The hemoglobin subunit beta is coded by ''HBB'' gene which is on chromosome 11 . The amino acid sequences of the globin proteins in hemoglobins usually differ between species. These differences grow with evolutionary distance between species. For example, the most common hemoglobin sequences in humans, bonobos and chimpanzees are completely identical, without even a single amino acid difference in either the alpha or the beta globin protein chains. Whereas the human and gorilla hemoglobin differ in one amino acid in both alpha and beta chains, these differences grow larger between less closely related species.
Even within species, variants of hemoglobin exist, although one sequence is usually "most common" in each species. Mutations
In biology, a mutation is an alteration in the nucleic acid sequence of the genome of an organism, virus, or extrachromosomal DNA. Viral genomes contain either DNA or RNA. Mutations result from errors during DNA or viral replication, mi ...
in the genes
In biology, the word gene (from , ; "...Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a ba ...
for the hemoglobin protein in a species result in hemoglobin variants. Many of these mutant forms of hemoglobin cause no disease. Some of these mutant forms of hemoglobin, however, cause a group of hereditary diseases termed the '' hemoglobinopathies''. The best known hemoglobinopathy is sickle-cell disease, which was the first human disease whose mechanism was understood at the molecular level. A (mostly) separate set of diseases called thalassemia
Thalassemias are inherited blood disorders characterized by decreased hemoglobin production. Symptoms depend on the type and can vary from none to severe. Often there is mild to severe anemia (low red blood cells or hemoglobin). Anemia can result ...
s involves underproduction of normal and sometimes abnormal hemoglobins, through problems and mutations in globin gene regulation. All these diseases produce anemia.
 Variations in hemoglobin amino acid sequences, as with other proteins, may be adaptive. For example, hemoglobin has been found to adapt in different ways to high altitudes. Organisms living at high elevations experience lower partial pressures of oxygen compared to those at sea level. This presents a challenge to the organisms that inhabit such environments because hemoglobin, which normally binds oxygen at high partial pressures of oxygen, must be able to bind oxygen when it is present at a lower pressure. Different organisms have adapted to such a challenge. For example, recent studies have suggested genetic variants in deer mice that help explain how deer mice that live in the mountains are able to survive in the thin air that accompanies high altitudes. A researcher from the University of Nebraska-Lincoln found mutations in four different genes that can account for differences between deer mice that live in lowland prairies versus the mountains. After examining wild mice captured from both highlands and lowlands, it was found that: the genes of the two breeds are "virtually identical—except for those that govern the oxygen-carrying capacity of their hemoglobin". "The genetic difference enables highland mice to make more efficient use of their oxygen", since less is available at higher altitudes, such as those in the mountains. Mammoth hemoglobin featured mutations that allowed for oxygen delivery at lower temperatures, thus enabling mammoths to migrate to higher latitudes during the Pleistocene. This was also found in hummingbirds that inhabit the Andes. Hummingbirds already expend a lot of energy and thus have high oxygen demands and yet Andean hummingbirds have been found to thrive in high altitudes. Non-synonymous mutations in the hemoglobin gene of multiple species living at high elevations (''Oreotrochilus, A. castelnaudii, C. violifer, P. gigas,'' and ''A. viridicuada'') have caused the protein to have less of an affinity for inositol hexaphosphate (IHP), a molecule found in birds that has a similar role as 2,3-BPG in humans; this results in the ability to bind oxygen in lower partial pressures.
Birds' unique circulatory lungs also promote efficient use of oxygen at low partial pressures of O2. These two adaptations reinforce each other and account for birds' remarkable high-altitude performance.
Hemoglobin adaptation extends to humans, as well. There is a higher offspring survival rate among Tibetan women with high oxygen saturation genotypes residing at 4,000 m. Natural selection seems to be the main force working on this gene because the mortality rate of offspring is significantly lower for women with higher hemoglobin-oxygen affinity when compared to the mortality rate of offspring from women with low hemoglobin-oxygen affinity. While the exact genotype and mechanism by which this occurs is not yet clear, selection is acting on these women's ability to bind oxygen in low partial pressures, which overall allows them to better sustain crucial metabolic processes.
Variations in hemoglobin amino acid sequences, as with other proteins, may be adaptive. For example, hemoglobin has been found to adapt in different ways to high altitudes. Organisms living at high elevations experience lower partial pressures of oxygen compared to those at sea level. This presents a challenge to the organisms that inhabit such environments because hemoglobin, which normally binds oxygen at high partial pressures of oxygen, must be able to bind oxygen when it is present at a lower pressure. Different organisms have adapted to such a challenge. For example, recent studies have suggested genetic variants in deer mice that help explain how deer mice that live in the mountains are able to survive in the thin air that accompanies high altitudes. A researcher from the University of Nebraska-Lincoln found mutations in four different genes that can account for differences between deer mice that live in lowland prairies versus the mountains. After examining wild mice captured from both highlands and lowlands, it was found that: the genes of the two breeds are "virtually identical—except for those that govern the oxygen-carrying capacity of their hemoglobin". "The genetic difference enables highland mice to make more efficient use of their oxygen", since less is available at higher altitudes, such as those in the mountains. Mammoth hemoglobin featured mutations that allowed for oxygen delivery at lower temperatures, thus enabling mammoths to migrate to higher latitudes during the Pleistocene. This was also found in hummingbirds that inhabit the Andes. Hummingbirds already expend a lot of energy and thus have high oxygen demands and yet Andean hummingbirds have been found to thrive in high altitudes. Non-synonymous mutations in the hemoglobin gene of multiple species living at high elevations (''Oreotrochilus, A. castelnaudii, C. violifer, P. gigas,'' and ''A. viridicuada'') have caused the protein to have less of an affinity for inositol hexaphosphate (IHP), a molecule found in birds that has a similar role as 2,3-BPG in humans; this results in the ability to bind oxygen in lower partial pressures.
Birds' unique circulatory lungs also promote efficient use of oxygen at low partial pressures of O2. These two adaptations reinforce each other and account for birds' remarkable high-altitude performance.
Hemoglobin adaptation extends to humans, as well. There is a higher offspring survival rate among Tibetan women with high oxygen saturation genotypes residing at 4,000 m. Natural selection seems to be the main force working on this gene because the mortality rate of offspring is significantly lower for women with higher hemoglobin-oxygen affinity when compared to the mortality rate of offspring from women with low hemoglobin-oxygen affinity. While the exact genotype and mechanism by which this occurs is not yet clear, selection is acting on these women's ability to bind oxygen in low partial pressures, which overall allows them to better sustain crucial metabolic processes.
Synthesis
Hemoglobin (Hb) is synthesized in a complex series of steps. The heme part is synthesized in a series of steps in themitochondria
A mitochondrion (; ) is an organelle found in the Cell (biology), cells of most Eukaryotes, such as animals, plants and Fungus, fungi. Mitochondria have a double lipid bilayer, membrane structure and use aerobic respiration to generate adenosi ...
and the cytosol of immature red blood cells, while the globin protein parts are synthesized by ribosome
Ribosomes ( ) are macromolecular machines, found within all cells, that perform biological protein synthesis (mRNA translation). Ribosomes link amino acids together in the order specified by the codons of messenger RNA (mRNA) molecules to ...
s in the cytosol. Production of Hb continues in the cell throughout its early development from the proerythroblast to the reticulocyte in the bone marrow
Bone marrow is a semi-solid tissue found within the spongy (also known as cancellous) portions of bones. In birds and mammals, bone marrow is the primary site of new blood cell production (or haematopoiesis). It is composed of hematopoietic ce ...
. At this point, the nucleus is lost in mammalian red blood cells, but not in birds and many other species. Even after the loss of the nucleus in mammals, residual ribosomal RNA allows further synthesis of Hb until the reticulocyte loses its RNA soon after entering the vasculature (this hemoglobin-synthetic RNA in fact gives the reticulocyte its reticulated appearance and name).
Structure of heme
Hemoglobin has a quaternary structure characteristic of many multi-subunit globular proteins. Most of the amino acids in hemoglobin form alpha helices, and these helices are connected by short non-helical segments. Hydrogen bonds stabilize the helical sections inside this protein, causing attractions within the molecule, which then causes each polypeptide chain to fold into a specific shape. Hemoglobin's quaternary structure comes from its four subunits in roughly a tetrahedral arrangement. In most vertebrates, the hemoglobin molecule is an assembly of four globular protein subunits. Each subunit is composed of a protein chain tightly associated with a non-protein prosthetic heme group. Each protein chain arranges into a set ofalpha-helix
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues ear ...
structural segments connected together in a globin fold arrangement. Such a name is given because this arrangement is the same folding motif used in other heme/globin proteins such as myoglobin
Myoglobin (symbol Mb or MB) is an iron- and oxygen-binding protein found in the cardiac and skeletal muscle tissue of vertebrates in general and in almost all mammals. Myoglobin is distantly related to hemoglobin. Compared to hemoglobin, myoglobi ...
. This folding pattern contains a pocket that strongly binds the heme group.
A heme group consists of an iron (Fe) ion held in a heterocyclic ring, known as a porphyrin. This porphyrin ring consists of four pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-meth ...
molecules cyclically linked together (by methine bridges) with the iron ion bound in the center. The iron ion, which is the site of oxygen binding, coordinates with the four nitrogen atoms in the center of the ring, which all lie in one plane. The heme is bound strongly (covalently) to the globular protein via the N atoms of the imidazole ring of F8 histidine residue (also known as the proximal histidine) below the porphyrin ring. A sixth position can reversibly bind oxygen by a coordinate covalent bond
In coordination chemistry, a coordinate covalent bond, also known as a dative bond, dipolar bond, or coordinate bond is a kind of two-center, two-electron covalent bond in which the two electrons derive from the same atom. The bonding of metal io ...
, completing the octahedral group of six ligands. This reversible bonding with oxygen is why hemoglobin is so useful for transporting oxygen around the body. Oxygen binds in an "end-on bent" geometry where one oxygen atom binds to Fe and the other protrudes at an angle. When oxygen is not bound, a very weakly bonded water molecule fills the site, forming a distorted octahedron.
Even though carbon dioxide is carried by hemoglobin, it does not compete with oxygen for the iron-binding positions but is bound to the amine groups of the protein chains attached to the heme groups.
The iron ion may be either in the ferrous Fe2+ or in the ferric Fe3+ state, but ferrihemoglobin ( methemoglobin) (Fe3+) cannot bind oxygen. In binding, oxygen temporarily and reversibly oxidizes (Fe2+) to (Fe3+) while oxygen temporarily turns into the superoxide ion, thus iron must exist in the +2 oxidation state to bind oxygen. If superoxide ion associated to Fe3+ is protonated, the hemoglobin iron will remain oxidized and incapable of binding oxygen. In such cases, the enzyme methemoglobin reductase
Cytochrome-''b''5 reductase is a NADH-dependent enzyme that converts ferricytochrome from a Fe3+ form to a Fe2+ form. It contains FAD and catalyzes the reaction:
In its b5-reducing capacity, this enzyme is involved in desaturation and elongatio ...
will be able to eventually reactivate methemoglobin by reducing the iron center.
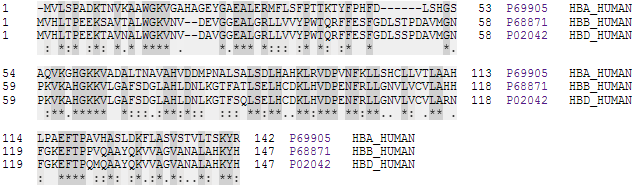
In adult humans, the most common hemoglobin type is a tetramer (which contains four subunit proteins) called ''hemoglobin A'', consisting of two α and two β subunits non-covalently bound, each made of 141 and 146 amino acid residues, respectively. This is denoted as α2β2. The subunits are structurally similar and about the same size. Each subunit has a molecular weight of about 16,000 daltons, for a total molecular weight of the tetramer of about 64,000 daltons (64,458 g/mol). Thus, 1 g/dL = 0.1551 mmol/L. Hemoglobin A is the most intensively studied of the hemoglobin molecules.
In human infants, the hemoglobin molecule is made up of 2 α chains and 2 γ chains. The γ chains are gradually replaced by β chains as the infant grows."Hemoglobin."MedicineNet. Web. 12 Oct. 2009. The four polypeptide chains are bound to each other by salt bridges,
hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
s, and the hydrophobic effect.
Oxygen saturation
In general, hemoglobin can be saturated with oxygen molecules (oxyhemoglobin), or desaturated with oxygen molecules (deoxyhemoglobin).Oxyhemoglobin
''Oxyhemoglobin'' is formed during physiological respiration when oxygen binds to the heme component of the protein hemoglobin in red blood cells. This process occurs in the pulmonary capillaries adjacent to the alveoli of the lungs. The oxygen then travels through the blood stream to be dropped off at cells where it is utilized as a terminal electron acceptor in the production ofATP
ATP may refer to:
Companies and organizations
* Association of Tennis Professionals, men's professional tennis governing body
* American Technical Publishers, employee-owned publishing company
* ', a Danish pension
* Armenia Tree Project, non ...
by the process of oxidative phosphorylation
Oxidative phosphorylation (UK , US ) or electron transport-linked phosphorylation or terminal oxidation is the metabolic pathway in which cells use enzymes to oxidize nutrients, thereby releasing chemical energy in order to produce adenosine tri ...
. It does not, however, help to counteract a decrease in blood pH. Ventilation, or breathing, may reverse this condition by removal of carbon dioxide, thus causing a shift up in pH.
Hemoglobin exists in two forms, a ''taut (tense) form'' (T) and a ''relaxed form'' (R). Various factors such as low pH, high CO2 and high 2,3 BPG
2,3-Bisphosphoglyceric acid (conjugate base 2,3-bisphosphoglycerate) (2,3-BPG), also known as 2,3-diphosphoglyceric acid (conjugate base 2,3-diphosphoglycerate) (2,3-DPG), is a three-carbon isomer of the glycolytic intermediate 1,3-bisphosphoglyc ...
at the level of the tissues favor the taut form, which has low oxygen affinity and releases oxygen in the tissues. Conversely, a high pH, low CO2, or low 2,3 BPG favors the relaxed form, which can better bind oxygen. The partial pressure of the system also affects O2 affinity where, at high partial pressures of oxygen (such as those present in the alveoli), the relaxed (high affinity, R) state is favoured. Inversely, at low partial pressures (such as those present in respiring tissues), the (low affinity, T) tense state is favoured. Additionally, the binding of oxygen to the iron(II) heme pulls the iron into the plane of the porphyrin ring, causing a slight conformational shift. The shift encourages oxygen to bind to the three remaining heme units within hemoglobin (thus, oxygen binding is cooperative).
Deoxygenated hemoglobin
Deoxygenated hemoglobin (deoxyhemoglobin) is the form of hemoglobin without the bound oxygen. The absorption spectra of oxyhemoglobin and deoxyhemoglobin differ. The oxyhemoglobin has significantly lower absorption of the 660 nm wavelength than deoxyhemoglobin, while at 940 nm its absorption is slightly higher. This difference is used for the measurement of the amount of oxygen in a patient's blood by an instrument called a pulse oximeter. This difference also accounts for the presentation ofcyanosis
Cyanosis is the change of body tissue color to a bluish-purple hue as a result of having decreased amounts of oxygen bound to the hemoglobin in the red blood cells of the capillary bed. Body tissues that show cyanosis are usually in locations ...
, the blue to purplish color that tissues develop during hypoxia
Hypoxia means a lower than normal level of oxygen, and may refer to:
Reduced or insufficient oxygen
* Hypoxia (environmental), abnormally low oxygen content of the specific environment
* Hypoxia (medical), abnormally low level of oxygen in the tis ...
.
Deoxygenated hemoglobin is paramagnetic; it is weakly attracted to magnetic fields. In contrast, oxygenated hemoglobin exhibits diamagnetism, a weak repulsion from a magnetic field.
Evolution of vertebrate hemoglobin
Scientists agree that the event that separated myoglobin from hemoglobin occurred after lampreys diverged from jawed vertebrates. This separation of myoglobin and hemoglobin allowed for the different functions of the two molecules to arise and develop: myoglobin has more to do with oxygen storage while hemoglobin is tasked with oxygen transport. The α- and β-like globin genes encode the individual subunits of the protein. The predecessors of these genes arose through another duplication event also after the gnathosome common ancestor derived from jawless fish, approximately 450–500 million years ago. Ancestral reconstruction studies suggest that the preduplication ancestor of the α and β genes was a dimer made up of identical globin subunits, which then evolved to assemble into a tetrameric architecture after the duplication. The development of α and β genes created the potential for hemoglobin to be composed of multiple distinct subunits, a physical composition central to hemoglobin's ability to transport oxygen. Having multiple subunits contributes to hemoglobin's ability to bind oxygen cooperatively as well as be regulated allosterically. Subsequently, the α gene also underwent a duplication event to form the ''HBA1'' and ''HBA2'' genes. These further duplications and divergences have created a diverse range of α- and β-like globin genes that are regulated so that certain forms occur at different stages of development. Most ice fish of the family Channichthyidae have lost their hemoglobin genes as an adaptation to cold water.Iron's oxidation state in oxyhemoglobin
Assigning oxygenated hemoglobin's oxidation state is difficult because oxyhemoglobin (Hb-O2), by experimental measurement, is diamagnetic (no net unpaired electrons), yet the lowest-energy (ground-state) electron configurations in both oxygen and iron are paramagnetic (suggesting at least one unpaired electron in the complex). The lowest-energy form of oxygen, and the lowest energy forms of the relevant oxidation states of iron, are these: * Triplet oxygen, the lowest-energy molecular oxygen species, has two unpaired electrons in antibonding π* molecular orbitals. * Iron(II) tends to exist in a high-spin 3d6 configuration with four unpaired electrons. * Iron(III) (3d5) has an odd number of electrons, and thus must have one or more unpaired electrons, in any energy state. All of these structures are paramagnetic (have unpaired electrons), not diamagnetic. Thus, a non-intuitive (e.g., a higher-energy for at least one species) distribution of electrons in the combination of iron and oxygen must exist, in order to explain the observed diamagnetism and no unpaired electrons. The two logical possibilities to produce diamagnetic (no net spin) Hb-O2 are: # Low-spin Fe2+ binds tosinglet oxygen
Singlet oxygen, systematically named dioxygen(singlet) and dioxidene, is a gaseous inorganic chemical with the formula O=O (also written as or ), which is in a quantum state where all electrons are spin paired. It is kinetically unstable at ambie ...
. Both low-spin iron and singlet oxygen are diamagnetic. However, the singlet form of oxygen is the higher-energy form of the molecule.
# Low-spin Fe3+ binds to O2•− (the superoxide ion) and the two unpaired electrons couple antiferromagnetically, giving observed diamagnetic properties. Here, the iron has been oxidized (has lost one electron), and the oxygen has been reduced (has gained one electron).
Another possible model in which low-spin Fe4+ binds to peroxide, O22−, can be ruled out by itself, because the iron is paramagnetic (although the peroxide ion is diamagnetic). Here, the iron has been oxidized by two electrons, and the oxygen reduced by two electrons.
Direct experimental data:
* X-ray photoelectron spectroscopy suggests iron has an oxidation state of approximately 3.2.
* Infrared vibrational frequencies of the O-O bond suggests a bond length fitting with superoxide (a bond order of about 1.6, with superoxide being 1.5).
* X-ray Absorption Near Edge Structures at the iron K-edge. The energy shift of 5 eV between deoxyhemoglobin and oxyhemoglobin, as for all the methemoglobin species, strongly suggests an actual local charge closer to Fe3+ than Fe2+.
Thus, the nearest formal oxidation state of iron in Hb-O2 is the +3 state, with oxygen in the −1 state (as superoxide .O2−). The diamagnetism in this configuration arises from the single unpaired electron on superoxide aligning antiferromagnetically with the single unpaired electron on iron (in a low-spin d5 state), to give no net spin to the entire configuration, in accordance with diamagnetic oxyhemoglobin from experiment.
The second choice of the logical possibilities above for diamagnetic oxyhemoglobin being found correct by experiment, is not surprising: singlet oxygen (possibility #1) is an unrealistically high energy state. Model 3 leads to unfavorable separation of charge (and does not agree with the magnetic data), although it could make a minor contribution as a resonance form. Iron's shift to a higher oxidation state in Hb-O2 decreases the atom's size, and allows it into the plane of the porphyrin ring, pulling on the coordinated histidine residue and initiating the allosteric changes seen in the globulins.
Early postulates by bio-inorganic chemists claimed that possibility #1 (above) was correct and that iron should exist in oxidation state II. This conclusion seemed likely, since the iron oxidation state III as methemoglobin, when ''not'' accompanied by superoxide .O2− to "hold" the oxidation electron, was known to render hemoglobin incapable of binding normal triplet O2 as it occurs in the air. It was thus assumed that iron remained as Fe(II) when oxygen gas was bound in the lungs. The iron chemistry in this previous classical model was elegant, but the required presence of the diamagnetic, high-energy, singlet oxygen molecule was never explained. It was classically argued that the binding of an oxygen molecule placed high-spin iron(II) in an octahedral field of strong-field ligands; this change in field would increase the crystal field splitting energy, causing iron's electrons to pair into the low-spin configuration, which would be diamagnetic in Fe(II). This forced low-spin pairing is indeed thought to happen in iron when oxygen binds, but is not enough to explain iron's change in size. Extraction of an additional electron from iron by oxygen is required to explain both iron's smaller size and observed increased oxidation state, and oxygen's weaker bond.
The assignment of a whole-number oxidation state is a formalism, as the covalent bonds are not required to have perfect bond orders involving whole electron transfer. Thus, all three models for paramagnetic Hb-O2 may contribute to some small degree (by resonance) to the actual electronic configuration of Hb-O2. However, the model of iron in Hb-O2 being Fe(III) is more correct than the classical idea that it remains Fe(II).
Cooperativity
When oxygen binds to the iron complex, it causes the iron atom to move back toward the center of the plane of the porphyrin ring (see moving diagram). At the same time, the imidazole side-chain of the histidine residue interacting at the other pole of the iron is pulled toward the porphyrin ring. This interaction forces the plane of the ring sideways toward the outside of the tetramer, and also induces a strain in the protein helix containing the histidine as it moves nearer to the iron atom. This strain is transmitted to the remaining three monomers in the tetramer, where it induces a similar conformational change in the other heme sites such that binding of oxygen to these sites becomes easier. As oxygen binds to one monomer of hemoglobin, the tetramer's conformation shifts from the T (tense) state to the R (relaxed) state. This shift promotes the binding of oxygen to the remaining three monomer's heme groups, thus saturating the hemoglobin molecule with oxygen. In the tetrameric form of normal adult hemoglobin, the binding of oxygen is, thus, a cooperative process. The binding affinity of hemoglobin for oxygen is increased by the oxygen saturation of the molecule, with the first molecules of oxygen bound influencing the shape of the binding sites for the next ones, in a way favorable for binding. This positive cooperative binding is achieved throughsteric
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
conformational changes of the hemoglobin protein complex as discussed above; i.e., when one subunit protein in hemoglobin becomes oxygenated, a conformational or structural change in the whole complex is initiated, causing the other subunits to gain an increased affinity for oxygen. As a consequence, the oxygen binding curve of hemoglobin is sigmoidal, or ''S''-shaped, as opposed to the normal hyperbolic curve associated with noncooperative binding.
The dynamic mechanism of the cooperativity in hemoglobin and its relation with low-frequency resonance has been discussed.
Binding for ligands other than oxygen
Besides the oxygen ligand, which binds to hemoglobin in a cooperative manner, hemoglobin ligands also includecompetitive inhibitors
Competitive inhibition is interruption of a chemical pathway owing to one chemical substance inhibiting the effect of another by competing with it for binding or bonding. Any metabolic or chemical messenger system can potentially be affected ...
such as carbon monoxide (CO) and allosteric ligands such as carbon dioxide (CO2) and nitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its che ...
(NO). The carbon dioxide is bound to amino groups of the globin proteins to form carbaminohemoglobin; this mechanism is thought to account for about 10% of carbon dioxide transport in mammals. Nitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its che ...
can also be transported by hemoglobin; it is bound to specific thiol groups in the globin protein to form an S-nitrosothiol, which dissociates into free nitric oxide and thiol again, as the hemoglobin releases oxygen from its heme site. This nitric oxide transport to peripheral tissues is hypothesized to assist oxygen transport in tissues, by releasing vasodilatory nitric oxide to tissues in which oxygen levels are low.
Competitive
The binding of oxygen is affected by molecules such as carbon monoxide (for example, from tobacco smoking, exhaust gas, and incomplete combustion in furnaces). CO competes with oxygen at the heme binding site. Hemoglobin's binding affinity for CO is 250 times greater than its affinity for oxygen, meaning that small amounts of CO dramatically reduce hemoglobin's ability to deliver oxygen to the target tissue. Since carbon monoxide is a colorless, odorless and tasteless gas, and poses a potentially fatal threat, carbon monoxide detectors have become commercially available to warn of dangerous levels in residences. When hemoglobin combines with CO, it forms a very bright red compound called carboxyhemoglobin, which may cause the skin of CO poisoning victims to appear pink in death, instead of white or blue. When inspired air contains CO levels as low as 0.02%,headache
Headache is the symptom of pain in the face, head, or neck. It can occur as a migraine, tension-type headache, or cluster headache. There is an increased risk of depression in those with severe headaches.
Headaches can occur as a result ...
and nausea occur; if the CO concentration is increased to 0.1%, unconsciousness will follow. In heavy smokers, up to 20% of the oxygen-active sites can be blocked by CO.
In similar fashion, hemoglobin also has competitive binding affinity for cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of a ...
(CN−), sulfur monoxide (SO), and sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds lar ...
(S2−), including hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The unde ...
(H2S). All of these bind to iron in heme without changing its oxidation state, but they nevertheless inhibit oxygen-binding, causing grave toxicity.
The iron atom in the heme group must initially be in the ferrous (Fe2+) oxidation state to support oxygen and other gases' binding and transport (it temporarily switches to ferric during the time oxygen is bound, as explained above). Initial oxidation to the ferric (Fe3+) state without oxygen converts hemoglobin into "hemiglobin" or methemoglobin, which cannot bind oxygen. Hemoglobin in normal red blood cells is protected by a reduction system to keep this from happening. Nitric oxide is capable of converting a small fraction of hemoglobin to methemoglobin in red blood cells. The latter reaction is a remnant activity of the more ancient nitric oxide dioxygenase
Nitric oxide dioxygenase () is an enzyme that catalyzes the conversion of nitric oxide (NO) to nitrate (NO)
. The net reaction for the reaction catalyzed by nitric oxide dioxygenase is shown below:
* 2NO + 2O2 + NAD(P)H → 2NO3− + NAD(P)+ + H+
...
function of globins.
Allosteric
Carbon ''di''oxide occupies a different binding site on the hemoglobin. At tissues, where carbon dioxide concentration is higher, carbon dioxide binds to allosteric site of hemoglobin, facilitating unloading of oxygen from hemoglobin and ultimately its removal from the body after the oxygen has been released to tissues undergoing metabolism. This increased affinity for carbon dioxide by the venous blood is known as theBohr effect
The Bohr effect is a phenomenon first described in 1904 by the Danish physiologist Christian Bohr. Hemoglobin's oxygen binding affinity (see oxygen–haemoglobin dissociation curve) is inversely related both to acidity and to the concentration o ...
. Through the enzyme carbonic anhydrase
The carbonic anhydrases (or carbonate dehydratases) () form a family of enzymes that catalyze the interconversion between carbon dioxide and water and the dissociated ions of carbonic acid (i.e. bicarbonate and hydrogen ions). The active site ...
, carbon dioxide reacts with water to give carbonic acid, which decomposes into bicarbonate and proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
s:
:CO2 + H2O → H2CO3 → HCO3− + H+
Hence, blood with high carbon dioxide levels is also lower in pH (more acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
ic). Hemoglobin can bind protons and carbon dioxide, which causes a conformational change in the protein and facilitates the release of oxygen. Protons bind at various places on the protein, while carbon dioxide binds at the α-amino group. Carbon dioxide binds to hemoglobin and forms carbaminohemoglobin. This decrease in hemoglobin's affinity for oxygen by the binding of carbon dioxide and acid is known as the Bohr effect
The Bohr effect is a phenomenon first described in 1904 by the Danish physiologist Christian Bohr. Hemoglobin's oxygen binding affinity (see oxygen–haemoglobin dissociation curve) is inversely related both to acidity and to the concentration o ...
. The Bohr effect favors the T state rather than the R state. (shifts the O2-saturation curve to the ''right''). Conversely, when the carbon dioxide levels in the blood decrease (i.e., in the lung capillaries), carbon dioxide and protons are released from hemoglobin, increasing the oxygen affinity of the protein. A reduction in the total binding capacity of hemoglobin to oxygen (i.e. shifting the curve down, not just to the right) due to reduced pH is called the root effect. This is seen in bony fish.
It is necessary for hemoglobin to release the oxygen that it binds; if not, there is no point in binding it. The sigmoidal curve of hemoglobin makes it efficient in binding (taking up O2 in lungs), and efficient in unloading (unloading O2 in tissues).
In people acclimated to high altitudes, the concentration of 2,3-Bisphosphoglycerate
2,3-Bisphosphoglyceric acid (conjugate base 2,3-bisphosphoglycerate) (2,3-BPG), also known as 2,3-diphosphoglyceric acid (conjugate base 2,3-diphosphoglycerate) (2,3-DPG), is a three-carbon isomer of the glycolytic intermediate 1,3-bisphosphoglyc ...
(2,3-BPG) in the blood is increased, which allows these individuals to deliver a larger amount of oxygen to tissues under conditions of lower oxygen tension. This phenomenon, where molecule Y affects the binding of molecule X to a transport molecule Z, is called a ''heterotropic'' allosteric effect. Hemoglobin in organisms at high altitudes has also adapted such that it has less of an affinity for 2,3-BPG and so the protein will be shifted more towards its R state. In its R state, hemoglobin will bind oxygen more readily, thus allowing organisms to perform the necessary metabolic processes when oxygen is present at low partial pressures.
Animals other than humans use different molecules to bind to hemoglobin and change its O2 affinity under unfavorable conditions. Fish use both ATP
ATP may refer to:
Companies and organizations
* Association of Tennis Professionals, men's professional tennis governing body
* American Technical Publishers, employee-owned publishing company
* ', a Danish pension
* Armenia Tree Project, non ...
and GTP. These bind to a phosphate "pocket" on the fish hemoglobin molecule, which stabilizes the tense state and therefore decreases oxygen affinity. GTP reduces hemoglobin oxygen affinity much more than ATP, which is thought to be due to an extra hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
formed that further stabilizes the tense state. Under hypoxic conditions, the concentration of both ATP and GTP is reduced in fish red blood cells to increase oxygen affinity.
A variant hemoglobin, called fetal hemoglobin
Fetal hemoglobin, or foetal haemoglobin (also hemoglobin F, HbF, or α2γ2) is the main oxygen carrier protein in the human fetus. Hemoglobin F is found in fetal red blood cells, and is involved in transporting oxygen from the mother's bloodstream ...
(HbF, α2γ2), is found in the developing fetus, and binds oxygen with greater affinity than adult hemoglobin. This means that the oxygen binding curve for fetal hemoglobin is left-shifted (i.e., a higher percentage of hemoglobin has oxygen bound to it at lower oxygen tension), in comparison to that of adult hemoglobin. As a result, fetal blood in the placenta is able to take oxygen from maternal blood.
Hemoglobin also carries nitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its che ...
(NO) in the globin part of the molecule. This improves oxygen delivery in the periphery and contributes to the control of respiration. NO binds reversibly to a specific cysteine residue in globin; the binding depends on the state (R or T) of the hemoglobin. The resulting S-nitrosylated hemoglobin influences various NO-related activities such as the control of vascular resistance, blood pressure and respiration. NO is not released in the cytoplasm of red blood cells but transported out of them by an anion exchanger called AE1.
Types in humans
Hemoglobin variants are a part of the normal embryonic and fetal development. They may also be pathologic mutant forms of hemoglobin in a population, caused by variations in genetics. Some well-known hemoglobin variants, such as sickle-cell anemia, are responsible for diseases and are considered hemoglobinopathies. Other variants cause no detectable pathology, and are thus considered non-pathological variants. In theembryo
An embryo is an initial stage of development of a multicellular organism. In organisms that reproduce sexually, embryonic development is the part of the life cycle that begins just after fertilization of the female egg cell by the male spe ...
:
* Gower 1 (ζ2ε2)
* Gower 2 (α2ε2) ()
* Hemoglobin Portland I (ζ2γ2)
* Hemoglobin Portland II (ζ2β2).
In the fetus:
* Hemoglobin F (α2γ2) ().
After birth:
* Hemoglobin A (adult hemoglobin) (α2β2) () – The most common with a normal amount over 95%
* Hemoglobin A2 (α2δ2) – δ chain synthesis begins late in the third trimester and, in adults, it has a normal range of 1.5–3.5%
* Hemoglobin F (fetal hemoglobin) (α2γ2) – In adults Hemoglobin F is restricted to a limited population of red cells called F-cells. However, the level of Hb F can be elevated in persons with sickle-cell disease and beta-thalassemia.
Hemoglobin D-Punjab
Within the medical specialty of hematology, Hemoglobin D-Punjab is one of the sub-variants of Hemoglobin D, a variant of hemoglobin found in human blood. It is so named because of its higher prevalence in the Punjab region of India and Pakistan. I ...
– (α2βD2) – A variant form of hemoglobin.
* Hemoglobin H (β4) – A variant form of hemoglobin, formed by a tetramer of β chains, which may be present in variants of α thalassemia.
* Hemoglobin Barts (γ4) – A variant form of hemoglobin, formed by a tetramer of γ chains, which may be present in variants of α thalassemia.
* Hemoglobin S (α2βS2) – A variant form of hemoglobin found in people with sickle cell disease. There is a variation in the β-chain gene, causing a change in the properties of hemoglobin, which results in sickling of red blood cells.
* Hemoglobin C (α2βC2) – Another variant due to a variation in the β-chain gene. This variant causes a mild chronic hemolytic anemia.
* Hemoglobin E (α2βE2) – Another variant due to a variation in the β-chain gene. This variant causes a mild chronic hemolytic anemia.
* Hemoglobin AS – A heterozygous form causing sickle cell trait with one adult gene and one sickle cell disease gene
* Hemoglobin SC disease – A compound heterozygous form with one sickle gene and another encoding Hemoglobin C.
* Hemoglobin Hopkins-2
Hemoglobin Hopkins-2 (Hb Hop-2) is a mutation of the protein hemoglobin, which is responsible for the transportation of oxygen through the blood from the lungs to the musculature of the body in vertebrates. The specific mutation in Hemoglobin Ho ...
– A variant form of hemoglobin that is sometimes viewed in combination with Hemoglobin S to produce sickle cell disease.
Degradation in vertebrate animals
When red blood cells reach the end of their life due to aging or defects, they are removed from the circulation by the phagocytic activity of macrophages in the spleen or the liver or hemolyze within the circulation. Free hemoglobin is then cleared from the circulation via the hemoglobin transporter CD163, which is exclusively expressed on monocytes or macrophages. Within these cells the hemoglobin molecule is broken up, and the iron gets recycled. This process also produces one molecule of carbon monoxide for every molecule of heme degraded. Heme degradation is the only natural source of carbon monoxide in the human body, and is responsible for the normal blood levels of carbon monoxide in people breathing normal air. The other major final product of heme degradation isbilirubin
Bilirubin (BR) (Latin for "red bile") is a red-orange compound that occurs in the normal catabolic pathway that breaks down heme in vertebrates. This catabolism is a necessary process in the body's clearance of waste products that arise from the ...
. Increased levels of this chemical are detected in the blood if red blood cells are being destroyed more rapidly than usual. Improperly degraded hemoglobin protein or hemoglobin that has been released from the blood cells too rapidly can clog small blood vessels, especially the delicate blood filtering vessels of the kidneys, causing kidney damage. Iron is removed from heme and salvaged for later use, it is stored as hemosiderin or ferritin in tissues and transported in plasma by beta globulins as transferrins. When the porphyrin ring is broken up, the fragments are normally secreted as a yellow pigment called bilirubin, which is secreted into the intestines as bile. Intestines metabolise bilirubin into urobilinogen. Urobilinogen leaves the body in faeces, in a pigment called stercobilin. Globulin is metabolised into amino acids that are then released into circulation.
Diseases related to hemoglobin
Hemoglobin deficiency can be caused either by a decreased amount of hemoglobin molecules, as in anemia, or by decreased ability of each molecule to bind oxygen at the same partial pressure of oxygen. Hemoglobinopathies (genetic defects resulting in abnormal structure of the hemoglobin molecule) may cause both. In any case, hemoglobin deficiency decreasesblood oxygen-carrying capacity
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells. Blood in the cir ...
. Hemoglobin deficiency is, in general, strictly distinguished from hypoxemia, defined as decreased partial pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal gas ...
of oxygen in blood, although both are causes of hypoxia
Hypoxia means a lower than normal level of oxygen, and may refer to:
Reduced or insufficient oxygen
* Hypoxia (environmental), abnormally low oxygen content of the specific environment
* Hypoxia (medical), abnormally low level of oxygen in the tis ...
(insufficient oxygen supply to tissues).
Other common causes of low hemoglobin include loss of blood, nutritional deficiency, bone marrow problems, chemotherapy, kidney failure, or abnormal hemoglobin (such as that of sickle-cell disease).
The ability of each hemoglobin molecule to carry oxygen is normally modified by altered blood pH or CO2, causing an altered oxygen–hemoglobin dissociation curve. However, it can also be pathologically altered in, e.g., carbon monoxide poisoning.
Decrease of hemoglobin, with or without an absolute decrease of red blood cells, leads to symptoms of anemia. Anemia has many different causes, although iron deficiency and its resultant iron deficiency anemia are the most common causes in the Western world. As absence of iron decreases heme synthesis, red blood cells in iron deficiency anemia are ''hypochromic'' (lacking the red hemoglobin pigment) and ''microcytic'' (smaller than normal). Other anemias are rarer. In hemolysis (accelerated breakdown of red blood cells), associated jaundice
Jaundice, also known as icterus, is a yellowish or greenish pigmentation of the skin and sclera due to high bilirubin levels. Jaundice in adults is typically a sign indicating the presence of underlying diseases involving abnormal heme meta ...
is caused by the hemoglobin metabolite bilirubin, and the circulating hemoglobin can cause kidney failure.
Some mutations in the globin chain are associated with the hemoglobinopathies, such as sickle-cell disease and thalassemia
Thalassemias are inherited blood disorders characterized by decreased hemoglobin production. Symptoms depend on the type and can vary from none to severe. Often there is mild to severe anemia (low red blood cells or hemoglobin). Anemia can result ...
. Other mutations, as discussed at the beginning of the article, are benign and are referred to merely as hemoglobin variants.
There is a group of genetic disorders, known as the '' porphyrias'' that are characterized by errors in metabolic pathways of heme synthesis. King George III of the United Kingdom
George III (George William Frederick; 4 June 173829 January 1820) was King of Great Britain and of Monarchy of Ireland, Ireland from 25 October 1760 until Acts of Union 1800, the union of the two kingdoms on 1 January 1801, after which he was ...
was probably the most famous porphyria sufferer.
To a small extent, hemoglobin A slowly combines with glucose at the terminal valine (an alpha aminoacid) of each β chain. The resulting molecule is often referred to as Hb A1c, a glycated hemoglobin. The binding of glucose to amino acids in the hemoglobin takes place spontaneously (without the help of an enzyme) in many proteins, and is not known to serve a useful purpose. However, as the concentration of glucose in the blood increases, the percentage of Hb A that turns into Hb A1c increases. In diabetics whose glucose usually runs high, the percent Hb A1c also runs high. Because of the slow rate of Hb A combination with glucose, the Hb A1c percentage reflects a weighted average of blood glucose levels over the lifetime of red cells, which is approximately 120 days. The levels of glycated hemoglobin are therefore measured in order to monitor the long-term control of the chronic disease of type 2 diabetes mellitus (T2DM). Poor control of T2DM results in high levels of glycated hemoglobin in the red blood cells. The normal reference range is approximately 4.0–5.9%. Though difficult to obtain, values less than 7% are recommended for people with T2DM. Levels greater than 9% are associated with poor control of the glycated hemoglobin, and levels greater than 12% are associated with very poor control. Diabetics who keep their glycated hemoglobin levels close to 7% have a much better chance of avoiding the complications that may accompany diabetes (than those whose levels are 8% or higher). In addition, increased glycated of hemoglobin increases its affinity for oxygen, therefore preventing its release at the tissue and inducing a level of hypoxia in extreme cases.
Elevated levels of hemoglobin are associated with increased numbers or sizes of red blood cells, called polycythemia. This elevation may be caused by congenital heart disease, cor pulmonale, pulmonary fibrosis, too much erythropoietin
Erythropoietin (; EPO), also known as erythropoetin, haematopoietin, or haemopoietin, is a glycoprotein cytokine secreted mainly by the kidneys in response to cellular hypoxia; it stimulates red blood cell production (erythropoiesis) in the bo ...
, or polycythemia vera. High hemoglobin levels may also be caused by exposure to high altitudes, smoking, dehydration (artificially by concentrating Hb), advanced lung disease and certain tumors.
A recent study done in Pondicherry, India, shows its importance in coronary artery disease.
Diagnostic uses
Hemoglobin concentration measurement is among the most commonly performedblood test
A blood test is a laboratory analysis performed on a blood sample that is usually extracted from a vein in the arm using a hypodermic needle, or via fingerprick. Multiple tests for specific blood components, such as a glucose test or a cholester ...
s, usually as part of a complete blood count. For example, it is typically tested before or after blood donation
A blood donation occurs when a person voluntarily has blood drawn and used for blood transfusion, transfusions and/or made into biopharmaceutical medications by a process called Blood fractionation, fractionation (separation of whole blood com ...
. Results are reported in g/ L, g/ dL or mol/L. 1 g/dL equals about 0.6206 mmol/L, although the latter units are not used as often due to uncertainty regarding the polymeric state of the molecule. This conversion factor, using the single globin unit molecular weight of 16,000 Da, is more common for hemoglobin concentration in blood. For MCHC (mean corpuscular hemoglobin concentration) the conversion factor 0.155, which uses the tetramer weight of 64,500 Da, is more common. Normal levels are:
* Men: 13.8 to 18.0 g/dL (138 to 180 g/L, or 8.56 to 11.17 mmol/L)
* Women: 12.1 to 15.1 g/dL (121 to 151 g/L, or 7.51 to 9.37 mmol/L)
* Children: 11 to 16 g/dL (110 to 160 g/L, or 6.83 to 9.93 mmol/L)
* Pregnant women: 11 to 14 g/dL (110 to 140 g/L, or 6.83 to 8.69 mmol/L) (9.5 to 15 usual value during pregnancy)
Normal values of hemoglobin in the 1st and 3rd trimesters of pregnant women must be at least 11 g/dL and at least 10.5 g/dL during the 2nd trimester.
Dehydration or hyperhydration can greatly influence measured hemoglobin levels. Albumin can indicate hydration status.
If the concentration is below normal, this is called anemia. Anemias are classified by the size of red blood cells, the cells that contain hemoglobin in vertebrates. The anemia is called "microcytic" if red cells are small, "macrocytic" if they are large, and "normocytic" otherwise.
Hematocrit, the proportion of blood volume occupied by red blood cells, is typically about three times the hemoglobin concentration measured in g/dL. For example, if the hemoglobin is measured at 17 g/dL, that compares with a hematocrit of 51%.
Laboratory hemoglobin test methods require a blood sample (arterial, venous, or capillary) and analysis on hematology analyzer and CO-oximeter. Additionally, a new noninvasive hemoglobin (SpHb) test method called Pulse CO-Oximetry is also available with comparable accuracy to invasive methods.
Concentrations of oxy- and deoxyhemoglobin can be measured continuously, regionally and noninvasively using NIRS. NIRS can be used both on the head and on muscles. This technique is often used for research in e.g. elite sports training, ergonomics, rehabilitation, patient monitoring, neonatal research, functional brain monitoring, brain–computer interface, urology (bladder contraction), neurology (Neurovascular coupling) and more.
Hemoglobin mass can be measured in humans using the non-radioactive, carbon monoxide (CO) rebreathing technique that has been used for more than 100 years. With this technique, a small volume of pure CO gas is inhaled and rebreathed for a few minutes. During rebreathing, CO binds to hemoglobin present in red blood cells. Based on the increase in blood CO after the rebreathing period, the haemoglobin mass can be determined through the dilution principle. Although CO gas in large volumes is toxic to humans, the volume of CO used to assess blood volumes corresponds to what would be inhaled when smoking a cigarette. While researchers typically use custom-made rebreathing circuits, the Detalo Performance from Detalo Health has automated the procedure and made the measurement available to a larger group of users.
Long-term control of blood sugar concentration can be measured by the concentration of Hb A1c. Measuring it directly would require many samples because blood sugar levels vary widely through the day. Hb A1c is the product of the irreversible reaction of hemoglobin A with glucose. A higher glucose concentration results in more Hb A1c. Because the reaction is slow, the Hb A1c proportion represents glucose level in blood averaged over the half-life of red blood cells, is typically ~120 days. An Hb A1c proportion of 6.0% or less show good long-term glucose control, while values above 7.0% are elevated. This test is especially useful for diabetics.
The functional magnetic resonance imaging
Functional magnetic resonance imaging or functional MRI (fMRI) measures brain activity by detecting changes associated with blood flow. This technique relies on the fact that cerebral blood flow and neuronal activation are coupled. When an area o ...
(fMRI) machine uses the signal from deoxyhemoglobin, which is sensitive to magnetic fields since it is paramagnetic. Combined measurement with NIRS shows good correlation with both the oxy- and deoxyhemoglobin signal compared to the BOLD signal.
Athletic tracking and self tracking uses
Hemoglobin can be tracked noninvasively, to build an individual data set tracking the hemoconcentration and hemodilution effects of daily activities for better understanding of sports performance and training. Athletes are often concerned about endurance and intensity of exercise. The sensor uses light-emitting diodes that emit red and infrared light through the tissue to a light detector, which then sends a signal to a processor to calculate the absorption of light by the hemoglobin protein. This sensor is similar to a pulse oximeter, which consists of a small sensing device that clips to the finger.Analogues in non-vertebrate organisms
A variety of oxygen-transport and -binding proteins exist in organisms throughout the animal and plant kingdoms. Organisms including bacteria,protozoa
Protozoa (singular: protozoan or protozoon; alternative plural: protozoans) are a group of single-celled eukaryotes, either free-living or parasitic, that feed on organic matter such as other microorganisms or organic tissues and debris. Histo ...
ns, and fungi all have hemoglobin-like proteins whose known and predicted roles include the reversible binding of gaseous ligands. Since many of these proteins contain globins and the heme moiety
Moiety may refer to:
Chemistry
* Moiety (chemistry), a part or functional group of a molecule
** Moiety conservation, conservation of a subgroup in a chemical species
Anthropology
* Moiety (kinship), either of two groups into which a society is ...
(iron in a flat porphyrin support), they are often called hemoglobins, even if their overall tertiary structure is very different from that of vertebrate hemoglobin. In particular, the distinction of "myoglobin" and hemoglobin in lower animals is often impossible, because some of these organisms do not contain muscle
Skeletal muscles (commonly referred to as muscles) are organs of the vertebrate muscular system and typically are attached by tendons to bones of a skeleton. The muscle cells of skeletal muscles are much longer than in the other types of muscl ...
s. Or, they may have a recognizable separate circulatory system but not one that deals with oxygen transport (for example, many insects and other arthropods). In all these groups, heme/globin-containing molecules (even monomeric globin ones) that deal with gas-binding are referred to as oxyhemoglobins. In addition to dealing with transport and sensing of oxygen, they may also deal with NO, CO2, sulfide compounds, and even O2 scavenging in environments that must be anaerobic. They may even deal with detoxification of chlorinated materials in a way analogous to heme-containing P450 enzymes and peroxidases.
The structure of hemoglobins varies across species. Hemoglobin occurs in all kingdoms of organisms, but not in all organisms. Primitive species such as bacteria, protozoa, algae
Algae (; singular alga ) is an informal term for a large and diverse group of photosynthetic eukaryotic organisms. It is a polyphyletic grouping that includes species from multiple distinct clades. Included organisms range from unicellular mic ...
, and plants often have single-globin hemoglobins. Many nematode
The nematodes ( or grc-gre, Νηματώδη; la, Nematoda) or roundworms constitute the phylum Nematoda (also called Nemathelminthes), with plant-Parasitism, parasitic nematodes also known as eelworms. They are a diverse animal phylum inhab ...
worms, molluscs, and crustaceans contain very large multisubunit molecules, much larger than those in vertebrates. In particular, chimeric hemoglobins found in fungi and giant annelids may contain both globin and other types of proteins.
One of the most striking occurrences and uses of hemoglobin in organisms is in the giant tube worm
''Riftia pachyptila'', commonly known as the giant tube worm and less commonly known as the Giant beardworm, is a marine invertebrate in the phylum Annelida (formerly grouped in phylum Pogonophora and Vestimentifera) related to tube worms ...
(''Riftia pachyptila'', also called Vestimentifera), which can reach 2.4 meters length and populates ocean volcanic vents. Instead of a digestive tract, these worms contain a population of bacteria constituting half the organism's weight. The bacteria oxidize H2S from the vent with O2 from the water to produce energy to make food from H2O and CO2. The worms' upper end is a deep-red fan-like structure ("plume"), which extends into the water and absorbs H2S and O2 for the bacteria, and CO2 for use as synthetic raw material similar to photosynthetic plants. The structures are bright red due to their content of several extraordinarily complex hemoglobins that have up to 144 globin chains, each including associated heme structures. These hemoglobins are remarkable for being able to carry oxygen in the presence of sulfide, and even to carry sulfide, without being completely "poisoned" or inhibited by it as hemoglobins in most other species are.
Other oxygen-binding proteins
;Myoglobin
Myoglobin (symbol Mb or MB) is an iron- and oxygen-binding protein found in the cardiac and skeletal muscle tissue of vertebrates in general and in almost all mammals. Myoglobin is distantly related to hemoglobin. Compared to hemoglobin, myoglobi ...
: Found in the muscle tissue of many vertebrates, including humans, it gives muscle tissue a distinct red or dark gray color. It is very similar to hemoglobin in structure and sequence, but is not a tetramer; instead, it is a monomer that lacks cooperative binding. It is used to store oxygen rather than transport it.
; Hemocyanin: The second most common oxygen-transporting protein found in nature, it is found in the blood of many arthropods and molluscs. Uses copper prosthetic groups instead of iron heme groups and is blue in color when oxygenated.
; Hemerythrin: Some marine invertebrates and a few species of annelid use this iron-containing non-heme protein to carry oxygen in their blood. Appears pink/violet when oxygenated, clear when not.
; Chlorocruorin: Found in many annelids, it is very similar to erythrocruorin, but the heme group is significantly different in structure. Appears green when deoxygenated and red when oxygenated.
; Vanabins: Also known as ''vanadium
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer ( pas ...
chromagens'', they are found in the blood of sea squirts. They were once hypothesized to use the metal vanadium as an oxygen binding prosthetic group. However, although they do contain vanadium by preference, they apparently bind little oxygen, and thus have some other function, which has not been elucidated (sea squirts also contain some hemoglobin). They may act as toxins.
; Erythrocruorin: Found in many annelids, including earthworms, it is a giant free-floating blood protein containing many dozens—possibly hundreds—of iron- and heme-bearing protein subunits bound together into a single protein complex with a molecular mass greater than 3.5 million daltons.
;Pinnaglobin: Only seen in the mollusc '' Pinna nobilis''. Brown manganese-based porphyrin protein.
; Leghemoglobin: In leguminous plants, such as alfalfa or soybeans, the nitrogen fixing bacteria in the roots are protected from oxygen by this iron heme containing oxygen-binding protein. The specific enzyme protected is nitrogenase, which is unable to reduce nitrogen gas in the presence of free oxygen.
;Coboglobin A coboglobin is a synthetic compound, a metalloprotein chemically similar to hemoglobin or myoglobin but using the metal cobalt instead of iron (hence the name). Just like hemoglobin and myoglobin, the coboglobins are able to reversibly bind molecul ...
: A synthetic cobalt-based porphyrin. Coboprotein would appear colorless when oxygenated, but yellow when in veins.
Presence in nonerythroid cells
Some nonerythroid cells (i.e., cells other than the red blood cell line) contain hemoglobin. In the brain, these include the A9dopaminergic
Dopaminergic means "related to dopamine" (literally, "working on dopamine"), dopamine being a common neurotransmitter. Dopaminergic substances or actions increase dopamine-related activity in the brain. Dopaminergic brain pathways facilitate d ...
neurons in the substantia nigra
The substantia nigra (SN) is a basal ganglia structure located in the midbrain that plays an important role in reward and movement. ''Substantia nigra'' is Latin for "black substance", reflecting the fact that parts of the substantia nigra app ...
, astrocytes in the cerebral cortex and hippocampus, and in all mature oligodendrocytes. It has been suggested that brain hemoglobin in these cells may enable the "storage of oxygen to provide a homeostatic mechanism in anoxic conditions, which is especially important for A9 DA neurons that have an elevated metabolism with a high requirement for energy production". It has been noted further that "A9 dopaminergic
Dopaminergic means "related to dopamine" (literally, "working on dopamine"), dopamine being a common neurotransmitter. Dopaminergic substances or actions increase dopamine-related activity in the brain. Dopaminergic brain pathways facilitate d ...
neurons may be at particular risk since in addition to their high mitochondrial activity they are under intense oxidative stress caused by the production of hydrogen peroxide via autoxidation and/or monoamine oxidase (MAO)-mediated deamination of dopamine and the subsequent reaction of accessible ferrous iron to generate highly toxic hydroxyl radicals". This may explain the risk of these cells for degeneration in Parkinson's disease. The hemoglobin-derived iron in these cells is not the cause of the post-mortem darkness of these cells (origin of the Latin name, substantia ''nigra''), but rather is due to neuromelanin.
Outside the brain, hemoglobin has non-oxygen-carrying functions as an antioxidant
Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. This can lead to polymerization and other chain reactions. They are frequently added to industrial products, such as fuels and lubricant ...
and a regulator of iron metabolism in macrophage
Macrophages (abbreviated as M φ, MΦ or MP) ( el, large eaters, from Greek ''μακρός'' (') = large, ''φαγεῖν'' (') = to eat) are a type of white blood cell of the immune system that engulfs and digests pathogens, such as cancer cel ...
s, alveolar cells, and mesangial cells in the kidney.
In history, art and music
Historically, an association between the color of blood and rust occurs in the association of the planet Mars, with the Roman god of war, since the planet is an orange-red, which reminded the ancients of blood. Although the color of the planet is due to iron compounds in combination with oxygen in the Martian soil, it is a common misconception that the iron in hemoglobin and its oxides gives blood its red color. The color is actually due to the porphyrinmoiety
Moiety may refer to:
Chemistry
* Moiety (chemistry), a part or functional group of a molecule
** Moiety conservation, conservation of a subgroup in a chemical species
Anthropology
* Moiety (kinship), either of two groups into which a society is ...
of hemoglobin to which the iron is bound, not the iron itself, although the ligation and redox state of the iron can influence the pi to pi* or n to pi* electronic transitions of the porphyrin and hence its optical characteristics.
Artist Julian Voss-Andreae
Julian Voss-Andreae (born 15 August 1970) is a German sculptor living and working in the U.S.
Life
Voss-Andreae's full first name is Johann Julian, in honor of his ancestor, German pastor Johann Valentin Andreae.
According to an interview with th ...
created a sculpture called ''Heart of Steel (Hemoglobin)'' in 2005, based on the protein's backbone. The sculpture was made from glass and weathering steel. The intentional rusting of the initially shiny work of art mirrors hemoglobin's fundamental chemical reaction of oxygen binding to iron.
Montreal artist Nicolas Baier created ''Lustre (Hémoglobine)'', a sculpture in stainless steel that shows the structure of the hemoglobin molecule. It is displayed in the atrium of McGill University Health Centre's research centre in Montreal. The sculpture measures about 10 metres × 10 metres × 10 metres.
See also
* Carbaminohemoglobin (Hb associated with ) * Carboxyhemoglobin (Hb associated with CO) *Chlorophyll
Chlorophyll (also chlorophyl) is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words , ("pale green") and , ("leaf"). Chlorophyll allow plants to a ...
(Mg heme)
* Complete Blood Count
* Delta globin
* Hemoglobinometer
A hemoglobinometer or haemoglobinometer (British English) is a medical device used to measure hemoglobin concentration in blood. It can operate by spectrophotometric measurement of hemoglobin concentration. Portable hemoglobinometers provide ...
* Hemoprotein
* Methemoglobin (ferric Hb, or ferrihemoglobin)
* Oxyhemoglobin (with diatomic oxygen, colored blood-red)
* Vaska's complex – iridium organometallic complex notable for its ability to bind to O2 reversibly
* Tegillarca granosa
References
Further reading
* * * * * Hazelwood, Loren (2001) ''Can't Live Without It: The story of hemoglobin in sickness and in health'', Nova Science Publishers * *External links
*National Anemia Action Council
a
anemia.org
at ttps://www.life-of-science.net www.life-of-science.net
Animation of hemoglobin: from deoxy to oxy form
a
vimeo.com
{{Authority control Hemoglobins Equilibrium chemistry Respiratory physiology