|
Alpha Helices
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues earlier along the protein sequence. The alpha helix is also called a classic Pauling–Corey–Branson α-helix. The name 3.613-helix is also used for this type of helix, denoting the average number of residues per helical turn, with 13 atoms being involved in the ring formed by the hydrogen bond. Among types of local structure in proteins, the α-helix is the most extreme and the most predictable from sequence, as well as the most prevalent. Discovery In the early 1930s, William Astbury showed that there were drastic changes in the X-ray fiber diffraction of moist wool or hair fibers upon significant stretching. The data suggested that the unstretched fibers had a coiled molecular structure with a characteristic repeat of ≈. Astbu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polypeptide Forming An Alpha Helix, With Hydrogen Bonds In Magenta
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. A polypeptide is a longer, continuous, unbranched peptide chain. Hence, peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. A polypeptide that contains more than approximately 50 amino acids is known as a protein. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, or to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyclic pepti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maurice Loyal Huggins
Maurice Loyal Huggins (19 September 1897, Berkeley County, West Virginia – 17 December 1981) was a scientist who independently conceived the idea of hydrogen bonding and who was an early advocate for their role in stabilizing protein secondary structure. An important polymer theory, Flory–Huggins theory, is also named after him. Controversies over the hydrogen bond Huggins believed that he had been the first to suggest the concept of the hydrogen bond, while he was a student under G. N. Lewis at the Chemical Laboratory of the University of California, Berkeley. According to his account, he wrote a thesis in 1919 in which the H-bond was introduced and applied to tautomerism in acetoacetic acid. Unfortunately, no hard copy of the thesis remains. The first extant publication of the H-bond was that of Wendell Latimer and Worth Rodebush in 1920, who cite Huggins' unpublished work in a footnote. (They were fellow scientists at the Chemical Laboratory.) Structure of the pepti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid ( carbamic acid is unstable), with the chemical formula NH2‐ CH2‐ COOH. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (GGU, GGC, GGA, GGG). Glycine is integral to the formation of alpha-helices in secondary protein structure due to its compact form. For the same reason, it is the most abundant amino acid in collagen triple-helices. Glycine is also an inhibitory neurotransmitter – interference with its release within the spinal cord (such as during a '' Clostridium tetani'' infection) can cause spastic paralysis due to uninhibited muscle contraction. It is the only achiral proteinogenic amino acid. It can fit into hydrophilic or hydrophobic environments, due to its minimal side chain of only one hydrogen atom. History and etymology Glycine was discovered in 1820 by the French ch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
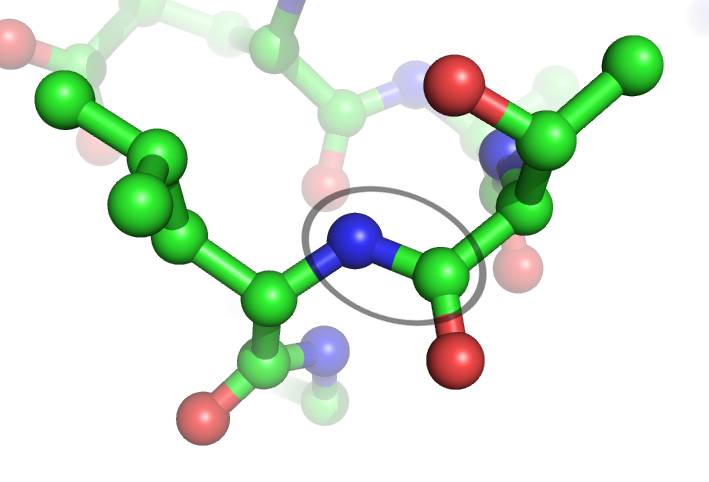
Peptide Bond
In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 ( nitrogen number two) of another, along a peptide or protein chain. It can also be called a eupeptide bond to distinguish it from an isopeptide bond, which is another type of amide bond between two amino acids. Synthesis When two amino acids form a '' dipeptide'' through a ''peptide bond'', it is a type of condensation reaction. In this kind of condensation, two amino acids approach each other, with the non- side chain (C1) carboxylic acid moiety of one coming near the non-side chain (N2) amino moiety of the other. One loses a hydrogen and oxygen from its carboxyl group (COOH) and the other loses a hydrogen from its amino group (NH2). This reaction produces a molecule of water (H2O) and two amino acids joined by a peptide bond (−CO−NH−). The two joined amino acids are called a dipeptide. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. A polypeptide is a longer, continuous, unbranched peptide chain. Hence, peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. A polypeptide that contains more than approximately 50 amino acids is known as a protein. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, or to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyclic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallography
Crystallography is the experimental science of determining the arrangement of atoms in crystalline solids. Crystallography is a fundamental subject in the fields of materials science and solid-state physics (condensed matter physics). The word "crystallography" is derived from the Greek word κρύσταλλος (''krystallos'') "clear ice, rock-crystal", with its meaning extending to all solids with some degree of transparency, and γράφειν (''graphein'') "to write". In July 2012, the United Nations recognised the importance of the science of crystallography by proclaiming that 2014 would be the International Year of Crystallography. denote a direction vector (in real space). * Coordinates in ''angle brackets'' or ''chevrons'' such as <100> denote a ''family'' of directions which are related by symmetry operations. In the cubic crystal system for example, would mean 00 10 01/nowiki> or the negative of any of those directions. * Miller indices in ''parentheses ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Keratin
Keratin () is one of a family of structural fibrous proteins also known as ''scleroproteins''. Alpha-keratin (α-keratin) is a type of keratin found in vertebrates. It is the key structural material making up scales, hair, nails, feathers, horns, claws, hooves, and the outer layer of skin among vertebrates. Keratin also protects epithelial cells from damage or stress. Keratin is extremely insoluble in water and organic solvents. Keratin monomers assemble into bundles to form intermediate filaments, which are tough and form strong unmineralized epidermal appendages found in reptiles, birds, amphibians, and mammals. Excessive keratinization participate in fortification of certain tissues such as in horns of cattle and rhinos, and armadillos' osteoderm. The only other biological matter known to approximate the toughness of keratinized tissue is chitin. Keratin comes in two types, the primitive, softer forms found in all vertebrates and harder, derived forms found only ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
William Lawrence Bragg
Sir William Lawrence Bragg, (31 March 1890 – 1 July 1971) was an Australian-born British physicist and X-ray crystallographer, discoverer (1912) of Bragg's law of X-ray diffraction, which is basic for the determination of crystal structure. He was joint recipient (with his father, William Henry Bragg) of the Nobel Prize in Physics in 1915, "For their services in the analysis of crystal structure by means of X-rays"; an important step in the development of X-ray crystallography. Bragg was knighted in 1941. As of 2021, he is the youngest ever Nobel laureate in physics, having received the award at the age of 25 years. Bragg was the director of the Cavendish Laboratory, Cambridge, when the discovery of the structure of DNA was reported by James D. Watson and Francis Crick in February 1953. Biography Early years Bragg was born in Adelaide, South Australia to Sir William Henry Bragg (1862–1942), Elder Professor of Mathematics and Physics at the University of Adelaide, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hugh Stott Taylor
Sir Hugh Stott Taylor (6 February 1890 – 17 April 1974) was an English chemist primarily interested in catalysis.Who Was Who, Published by A&C Black Limited In 1925, in a landmark contribution to catalytic theory, Taylor suggested that a catalysed chemical reaction is not catalysed over the entire solid surface of the catalyst but only at certain ' active sites' or centres. He also developed important methods for procuring heavy water during World War II and pioneered the use of stable isotopes in studying chemical reactions. Early life Taylor was born in St Helens, Lancashire, England in 1890, the son of glass technologist James and Ellen (née Stott) Taylor. He was educated at Cowley Grammar School in St Helens and then attended the University of Liverpool, where he received his BSc in 1909 and his MSc in 1910. Taylor then carried out three years of graduate work in Liverpool, after which he spent one year at the Nobel Institute in Stockholm in the laboratory of Svan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hans Neurath
Hans Neurath (October 29, 1909 – April 2002) was a biochemist, a leader in protein chemistry, and the founding chairman of the Department of Biochemistry at the University of Washington in Seattle. He was born in Vienna, Austria and received his doctorate in 1933 from the University of Vienna. He then studied in London and at the University of Minnesota. In 1938, he was appointed professor at Duke University, where he established a research program on the physical chemistry of proteins. Neurath was a member of the National Academy of Sciences and the American Academy of Arts and Sciences, and a foreign member of the Max Planck Society of Germany. Scientific research Neurath had wide-ranging interests in the physical chemistry of proteins. He published seminal papers on protein structure and denaturation and debunked early models of protein structures, notably those of William Astbury. His research focused mainly on the proteases, (proteins that act as enzymes cleaving other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Herman Branson
Herman Russell Branson (August 14, 1914 – June 7, 1995) was an American physicist, chemist, best known for his research on the alpha helix protein structure, and was also the president of two colleges. He received a fellowship from the Rosenwald Foundation. Early life Branson received his B.S. from Virginia State College in 1936, and his Ph.D. in physics from the University of Cincinnati, under the direction of Boris Podolsky, in 1939. His thesis was in three parts, the first involved the interaction of x-rays with Tubifex tubifex (or sludge worm), the second involving the design and construction of an X-ray intensity measuring device, and the third section on the quantization of mass using the Dirac Equation. After a stint at Dillard University, he joined Howard University in 1941 as an assistant professor of physics and chemistry. As a scientist, Branson made significant contributions to how proteins work, and how they contribute to diseases such as sickle cell anemia. H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Robert Corey
Robert Brainard Corey (August 19, 1897 – April 23, 1971) was an American biochemist, mostly known for his role in discovery of the α-helix and the β-sheet with Linus Pauling. Also working with Pauling was Herman Branson. Their discoveries were remarkably correct, with even the bond lengths being accurate until about 40 years later. The α-helix and β-sheet are two structures that are now known to form the backbones of many proteins. Academic training A childhood polio survivor, Corey received his undergraduate degree from the University of Pittsburgh, and his Ph.D. in chemistry from Cornell University (Marsh, p. 52-53). The findings of α-helix and β-sheet At Caltech, the trio (Pauling, Corey and Branson) published a series of 8 articles in the Proceedings of the National Academy of Sciences (PNAS). The most revolutionary of the 8 articles in PNAS is the one written on February 28, 1951. That date was also Pauling's 50th birthday. It was called "The Structure of P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |