|
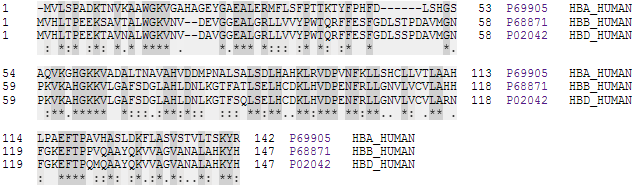
Hemoglobinopathy
Hemoglobinopathy is the medical term for a group of inherited blood disorders and diseases that primarily affect red blood cells. They are single-gene disorders and, in most cases, they are inherited as autosomal co-dominant traits. There are two main groups: abnormal structural hemoglobin variants caused by mutations in the hemoglobin genes, and the thalassemias, which are caused by an underproduction of otherwise normal hemoglobin molecules. The main structural hemoglobin variants are HbS, HbE and HbC. The main types of thalassemia are alpha-thalassemia and beta thalassemia. The two conditions may overlap because some conditions which cause abnormalities in hemoglobin proteins also affect their production. Some hemoglobin variants do not cause pathology or anemia, and thus are often not classed as hemoglobinopathies. Hemoglobin structural biology Normal human hemoglobins are tetrameric proteins composed of two pairs of globin chains, each of which contains one alpha-li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemoglobin M
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocytes) of almost all vertebrates (the exception being the fish family Channichthyidae) as well as the tissues of some invertebrates. Hemoglobin in blood carries oxygen from the respiratory organs (''e.g.'' lungs or gills) to the rest of the body (''i.e.'' tissues). There it releases the oxygen to permit aerobic respiration to provide energy to power functions of an organism in the process called metabolism. A healthy individual human has 12to 20grams of hemoglobin in every 100mL of blood. In mammals, the chromoprotein makes up about 96% of the red blood cells' dry content (by weight), and around 35% of the total content (including water). Hemoglobin has an oxygen-binding capacity of 1.34mL O2 per gram, which increases the total blood oxygen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemoglobin O
Hemoglobin O (HbO) is a rare type of hemoglobin in which there is a substitution of glutamic acid by lysine as in hemoglobin C, but at different positions. Since the amino acid substitution can occur at different positions of the β-globin chain of the protein, there are several variants. In hemoglobin O-Arab (HbO-Arab) substitution occurs at position 121, while in hemoglobin O-Padova (HbO-Padova) it is at 11 position, and in hemoglobin O Indonesia (HbOIna) it is at 116. HbO is usually harmless unlike other hemoglobin variants such as HbS and thalassemias, even under combination with these abnormal hemoglobins. Hemoglobin O-Padova is the most severe form and is associated with disease of the RBCs and spleen. __TOC__ Discovery Hemoglobin O Indonesia is the first discovered HbO. Lie-Injo Luan Eng at the University of Indonesia, Djakarta, was the first to notice the abnormal hemoglobin in 1956 among the Buginese people of Sulawesi Island in Indonesia. It was found among normal he ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood Disorder
Hematologic diseases are disorders which primarily affect the blood & blood-forming organs. Hematologic diseases include rare genetic disorders, anemia, HIV, sickle cell disease & complications from chemotherapy or transfusions. Myeloid * Hemoglobinopathies (congenital abnormality of the hemoglobin molecule or of the rate of hemoglobin synthesis) ** Sickle cell disease ** Thalassemia ** Methemoglobinemia * Anemias (lack of red blood cells or hemoglobin) ** Iron-deficiency anemia ** Megaloblastic anemia *** Vitamin B12 deficiency **** Pernicious anemia *** Folate deficiency ** Hemolytic anemias (destruction of red blood cells) *** Genetic disorders of RBC membrane **** Hereditary spherocytosis **** Hereditary elliptocytosis **** Congenital dyserythropoietic anemia *** Genetic disorders of RBC metabolism **** Glucose-6-phosphate dehydrogenase deficiency (G6PD) **** Pyruvate kinase deficiency *** Immune mediated hemolytic anemia ( direct Coombs test is positive) **** Autoimmune ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemoglobin Korle-Bu
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocytes) of almost all vertebrates (the exception being the fish family Channichthyidae) as well as the tissues of some invertebrates. Hemoglobin in blood carries oxygen from the respiratory organs (''e.g.'' lungs or gills) to the rest of the body (''i.e.'' tissues). There it releases the oxygen to permit aerobic respiration to provide energy to power functions of an organism in the process called metabolism. A healthy individual human has 12to 20grams of hemoglobin in every 100mL of blood. In mammals, the chromoprotein makes up about 96% of the red blood cells' dry content (by weight), and around 35% of the total content (including water). Hemoglobin has an oxygen-binding capacity of 1.34mL O2 per gram, which increases the total blood oxygen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemoglobin Hasharon
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocytes) of almost all vertebrates (the exception being the fish family Channichthyidae) as well as the tissues of some invertebrates. Hemoglobin in blood carries oxygen from the respiratory organs (''e.g.'' lungs or gills) to the rest of the body (''i.e.'' tissues). There it releases the oxygen to permit aerobic respiration to provide energy to power functions of an organism in the process called metabolism. A healthy individual human has 12to 20grams of hemoglobin in every 100mL of blood. In mammals, the chromoprotein makes up about 96% of the red blood cells' dry content (by weight), and around 35% of the total content (including water). Hemoglobin has an oxygen-binding capacity of 1.34mL O2 per gram, which increases the total blood oxygen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-thalassemia
Alpha-thalassemia (α-thalassemia, α-thalassaemia) is a form of thalassemia involving the genes '' HBA1'' and ''HBA2''. Thalassemias are a group of inherited blood conditions which result in the impaired production of hemoglobin, the molecule that carries oxygen in the blood. Normal hemoglobin consists of two alpha chains and two beta chains; in alpha-thalassemia, there is a quantitative decrease in the amount of alpha chains, resulting in fewer normal hemoglobin molecules. Furthermore, alpha-thalassemia leads to the production of unstable beta globin molecules which cause increased red blood cell destruction. The degree of impairment is based on which clinical phenotype is present (how many genes are affected). Signs and symptoms The presentation of individuals with alpha-thalassemia consists of: Cause Alpha-thalassemias are most commonly inherited in a Mendelian recessive manner. They are also associated with deletions of chromosome 16p. Alpha thalassemia can also be acq ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Thalassemia
Beta thalassemias (β thalassemias) are a group of inherited blood disorders. They are forms of thalassemia caused by reduced or absent synthesis of the beta chains of hemoglobin that result in variable outcomes ranging from severe anemia to clinically asymptomatic individuals. Global annual incidence is estimated at one in 100,000. Beta thalassemias occur due to malfunctions in the hemoglobin subunit beta or HBB. The severity of the disease depends on the nature of the mutation. HBB blockage over time leads to decreased beta-chain synthesis. The body's inability to construct new beta-chains leads to the underproduction of HbA (adult hemoglobin). Reductions in HbA available overall to fill the red blood cells in turn leads to microcytic anemia. Microcytic anemia ultimately develops in respect to inadequate HBB protein for sufficient red blood cell functioning. Due to this factor, the patient may require blood transfusions to make up for the blockage in the beta-chains. Repeat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemoglobin Constant Spring
Hemoglobin Constant Spring is a variant of hemoglobin in which a mutation in the alpha globin gene produces an alpha globin chain that is abnormally long. It is the most common nondeletional alpha-thalassemia mutation associated with hemoglobin H disease. The quantity of hemoglobin in the cells is low because the messenger RNA is unstable and some is degraded prior to protein synthesis. Another reason is that the Constant Spring alpha chain protein is itself unstable. The result is a thalassemic phenotype. Hemoglobin Constant Spring is renamed after Constant Spring district in Jamaica. See also * Hemoglobin variants * Hemoglobinopathy * Thalassemia Thalassemias are inherited blood disorders characterized by decreased hemoglobin production. Symptoms depend on the type and can vary from none to severe. Often there is mild to severe anemia (low red blood cells or hemoglobin). Anemia can resul ... References {{Reflist Hemoglobins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemoglobin A
Hemoglobin A (HbA), also known as adult hemoglobin, hemoglobin A1 or α2β2, is the most common human hemoglobin tetramer, accounting for over 97% of the total red blood cell hemoglobin. Hemoglobin is an oxygen-binding protein, found in erythrocytes, which transports oxygen from the lungs to the tissues. Hemoglobin A is the most common adult form of hemoglobin and exists as a tetramer containing two alpha subunits and two beta subunits (α2β2). Hemoglobin A2 (HbA2) is a less common adult form of hemoglobin and is composed of two alpha and two delta-globin subunits. This hemoglobin makes up 1-3% of hemoglobin in adults. Structure and function Hemoglobin A (HbA) is the most common adult form of hemoglobin and exists as a tetramer containing two alpha subunits and two beta subunits (α2β2). Each subunit contains a heme group that diatomic oxygen (O2) molecules can bind to. In addition to oxygen, subunit assembly and quaternary structure are known to play important roles in Hb a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemoglobin G
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocytes) of almost all vertebrates (the exception being the fish family Channichthyidae) as well as the tissues of some invertebrates. Hemoglobin in blood carries oxygen from the respiratory organs (''e.g.'' lungs or gills) to the rest of the body (''i.e.'' tissues). There it releases the oxygen to permit aerobic respiration to provide energy to power functions of an organism in the process called metabolism. A healthy individual human has 12to 20grams of hemoglobin in every 100mL of blood. In mammals, the chromoprotein makes up about 96% of the red blood cells' dry content (by weight), and around 35% of the total content (including water). Hemoglobin has an oxygen-binding capacity of 1.34mL O2 per gram, which increases the total blood oxygen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocytes) of almost all vertebrates (the exception being the fish family Channichthyidae) as well as the tissues of some invertebrates. Hemoglobin in blood carries oxygen from the respiratory organs (''e.g.'' lungs or gills) to the rest of the body (''i.e.'' tissues). There it releases the oxygen to permit aerobic respiration to provide energy to power functions of an organism in the process called metabolism. A healthy individual human has 12to 20grams of hemoglobin in every 100mL of blood. In mammals, the chromoprotein makes up about 96% of the red blood cells' dry content (by weight), and around 35% of the total content (including water). Hemoglobin has an oxygen-binding capacity of 1.34mL O2 per gram, which increases the total blood oxygen ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |