heat shock protein 70 on:
[Wikipedia]
[Google]
[Amazon]
 The 70 kilodalton heat shock proteins (Hsp70s or DnaK) are a
The 70 kilodalton heat shock proteins (Hsp70s or DnaK) are a
 The Hsp70 proteins have three major functional domains:
* N-terminal ATPase domain – binds ATP (
The Hsp70 proteins have three major functional domains:
* N-terminal ATPase domain – binds ATP (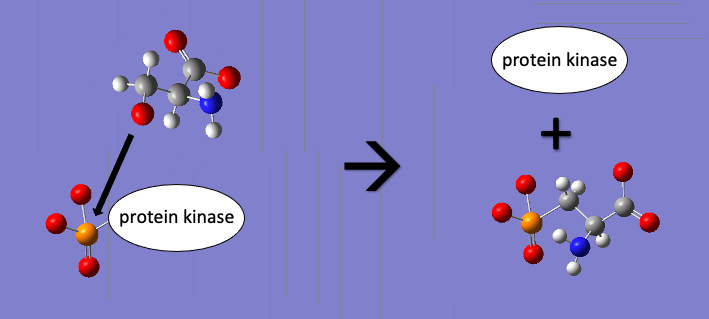 Protein phosphorylation, a post-translational modification, helps to regulate protein function and involves the phosphorylation of amino acids with hydroxyl groups in their side chains (among eukaryotes). Serine, threonine, and tyrosine amino acids are common targets of phosphorylation. Phosphorylation of Hsp70 has become a point of greater exploration in scientific literature relatively recently. A 2020 publication suggests that phosphorylation of a serine residue between the NBD and substrate binding domain in yeast Hsp70s leads to a dramatic reduction of the normal Hsp70 heat shock response. This deactivation via phosphorylation of a protein is a common motif in protein regulation, and demonstrates how relatively small changes to protein structure can have biologically significant effects on protein function.
Protein phosphorylation, a post-translational modification, helps to regulate protein function and involves the phosphorylation of amino acids with hydroxyl groups in their side chains (among eukaryotes). Serine, threonine, and tyrosine amino acids are common targets of phosphorylation. Phosphorylation of Hsp70 has become a point of greater exploration in scientific literature relatively recently. A 2020 publication suggests that phosphorylation of a serine residue between the NBD and substrate binding domain in yeast Hsp70s leads to a dramatic reduction of the normal Hsp70 heat shock response. This deactivation via phosphorylation of a protein is a common motif in protein regulation, and demonstrates how relatively small changes to protein structure can have biologically significant effects on protein function.
 By binding tightly to partially synthesized peptide sequences (incomplete proteins), Hsp70 prevents them from aggregating and being rendered nonfunctional. Once the entire protein is synthesized, a nucleotide exchange factor (prokaryotic
By binding tightly to partially synthesized peptide sequences (incomplete proteins), Hsp70 prevents them from aggregating and being rendered nonfunctional. Once the entire protein is synthesized, a nucleotide exchange factor (prokaryotic
 Hsp90s are essential for protein remodeling, similar to Hsp70 proteins, and play an especially vital role in eukaryotes, where it has been suggested that Hsp90 interacts with the DnaK system (composed of DnaK, GrpE, and either DnaJ or CbpA) to facilitate the process of protein remodeling. In E. coli, Hsp90s works collaboratively with Hsp70s to facilitate protein remodeling and activation. Hsp90Ec and DnaK are chaperones of Hsp90 and Hsp70, respectively. DnaK initially binds and stabilizes the misfolded protein before working collaboratively with Hsp90Ec to refold this substrate and cause its activation. Given conditions of excess DnaK, this chaperone has been found to inhibit remodeling of proteins. However, the presence of Hsp90Ec can mitigate this effect and enable protein remodeling despite conditions of excess DnaK.
The Hsp70 superfamily also includes a family of Hsp110/Grp170 (Sse) proteins, which are larger proteins related to Hsp70. The Hsp110 family of proteins have divergent functions: yeast Sse1p has little ATPase activity but is a chaperone on its own as well as a nucleotide exchange factor for Hsp70, while the closely related Sse2p has little unfoldase activity.
The following is a list of currently named human HSP110 genes. HSPH2-4 are proposed names and the current name is linked:
Hsp90s are essential for protein remodeling, similar to Hsp70 proteins, and play an especially vital role in eukaryotes, where it has been suggested that Hsp90 interacts with the DnaK system (composed of DnaK, GrpE, and either DnaJ or CbpA) to facilitate the process of protein remodeling. In E. coli, Hsp90s works collaboratively with Hsp70s to facilitate protein remodeling and activation. Hsp90Ec and DnaK are chaperones of Hsp90 and Hsp70, respectively. DnaK initially binds and stabilizes the misfolded protein before working collaboratively with Hsp90Ec to refold this substrate and cause its activation. Given conditions of excess DnaK, this chaperone has been found to inhibit remodeling of proteins. However, the presence of Hsp90Ec can mitigate this effect and enable protein remodeling despite conditions of excess DnaK.
The Hsp70 superfamily also includes a family of Hsp110/Grp170 (Sse) proteins, which are larger proteins related to Hsp70. The Hsp110 family of proteins have divergent functions: yeast Sse1p has little ATPase activity but is a chaperone on its own as well as a nucleotide exchange factor for Hsp70, while the closely related Sse2p has little unfoldase activity.
The following is a list of currently named human HSP110 genes. HSPH2-4 are proposed names and the current name is linked:
 The 70 kilodalton heat shock proteins (Hsp70s or DnaK) are a
The 70 kilodalton heat shock proteins (Hsp70s or DnaK) are a family
Family (from la, familia) is a group of people related either by consanguinity (by recognized birth) or affinity (by marriage or other relationship). The purpose of the family is to maintain the well-being of its members and of society. Idea ...
of conserved ubiquitously expressed heat shock protein
Heat shock proteins (HSP) are a family of proteins produced by cells in response to exposure to stressful conditions. They were first described in relation to heat shock, but are now known to also be expressed during other stresses including expo ...
s. Proteins with similar structure exist in virtually all living organisms. Intracellularly localized Hsp70s are an important part of the cell's machinery for protein folding
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduc ...
, performing chaperoning functions, and helping to protect cells from the adverse effects of physiological stress
Stress, either physiological, biological or psychological, is an organism's response to a stressor such as an environmental condition. Stress is the body's method of reacting to a condition such as a threat, challenge or physical and psycholo ...
es. Additionally, membrane-bound Hsp70s have been identified as a potential target for cancer therapies and their extracellularly localized counterparts have been identified as having both membrane-bound and membrane-free structures.
Discovery
Members of the Hsp70 family are very strongly upregulated by heat stress andtoxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subs ...
chemicals, particularly heavy metals such as arsenic, cadmium, copper, mercury, etc. Heat shock was originally discovered by Ferruccio Ritossa
Ferruccio Ritossa (February 26, 1936 – January 9, 2014) was an Italian geneticist best known for his discovery of the heat shock response in the model organism ''Drosophila'' (fruit flies).
Early life and education
Ritossa was born in the t ...
in the 1960s when a lab worker accidentally boosted the incubation temperature of Drosophila
''Drosophila'' () is a genus of flies, belonging to the family Drosophilidae, whose members are often called "small fruit flies" or (less frequently) pomace flies, vinegar flies, or wine flies, a reference to the characteristic of many speci ...
(fruit flies). When examining the chromosomes, Ritossa found a "puffing pattern" that indicated the elevated gene transcription of an unknown protein. This was later described as the "Heat Shock Response" and the proteins were termed the "Heat Shock Proteins" (Hsps).
Structure
 The Hsp70 proteins have three major functional domains:
* N-terminal ATPase domain – binds ATP (
The Hsp70 proteins have three major functional domains:
* N-terminal ATPase domain – binds ATP (Adenosine triphosphate
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms o ...
) and hydrolyzes it to ADP ( Adenosine diphosphate). The NBD (nucleotide binding domain) consists of two lobes with a deep cleft between them, at the bottom of which nucleotide (ATP and ADP) binds. The exchange of ATP and ADP leads to conformational changes in the other two domains.
* Substrate binding domain – is composed of a 15 kDa
The dalton or unified atomic mass unit (symbols: Da or u) is a non-SI unit of mass widely used in physics and chemistry. It is defined as of the mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state and at re ...
β sheet subdomain and a 10 kDa helical subdomain. The β sheet subdomain consists of stranded β sheets with upward protruding loops, as a typical β barrel, which enclose the peptide backbone of the substrate. SBD contains a groove with an affinity for neutral, hydrophobic amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
residues. The groove is long enough to interact with peptides up to seven residues in length.
* C-terminal
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is ...
domain – rich in alpha helical structure acts as a 'lid' for the substrate binding domain. The helical subdomain consists of five helices, with two helices packed against two sides of the β sheet subdomain, stabilizing the inner structure. In addition, one of the helix forms a salt bridge and several hydrogen bonds to the outer Loops, thereby closing the substrate-binding pocket like a lid. Three helices in this domain form another hydrophobic core which may be stabilization of the "lid". When an Hsp70 protein is ATP bound, the lid is open and peptides bind and release relatively rapidly. When Hsp70 proteins are ADP bound, the lid is closed, and peptides are tightly bound to the substrate binding domain.
 Protein phosphorylation, a post-translational modification, helps to regulate protein function and involves the phosphorylation of amino acids with hydroxyl groups in their side chains (among eukaryotes). Serine, threonine, and tyrosine amino acids are common targets of phosphorylation. Phosphorylation of Hsp70 has become a point of greater exploration in scientific literature relatively recently. A 2020 publication suggests that phosphorylation of a serine residue between the NBD and substrate binding domain in yeast Hsp70s leads to a dramatic reduction of the normal Hsp70 heat shock response. This deactivation via phosphorylation of a protein is a common motif in protein regulation, and demonstrates how relatively small changes to protein structure can have biologically significant effects on protein function.
Protein phosphorylation, a post-translational modification, helps to regulate protein function and involves the phosphorylation of amino acids with hydroxyl groups in their side chains (among eukaryotes). Serine, threonine, and tyrosine amino acids are common targets of phosphorylation. Phosphorylation of Hsp70 has become a point of greater exploration in scientific literature relatively recently. A 2020 publication suggests that phosphorylation of a serine residue between the NBD and substrate binding domain in yeast Hsp70s leads to a dramatic reduction of the normal Hsp70 heat shock response. This deactivation via phosphorylation of a protein is a common motif in protein regulation, and demonstrates how relatively small changes to protein structure can have biologically significant effects on protein function.
Function
The Hsp70 system interacts with extended peptide segments of proteins as well as partially folded proteins to cause aggregation of proteins in key pathways to downregulate activity. When not interacting with a substrate peptide, Hsp70 is usually in an ATP bound state. Hsp70 by itself is characterized by a very weak ATPase activity, such that spontaneous hydrolysis will not occur for many minutes. As newly synthesized proteins emerge from the ribosomes, the substrate binding domain of Hsp70 recognizes sequences of hydrophobic amino acid residues, and interacts with them. This spontaneous interaction is reversible, and in the ATP bound state Hsp70 may relatively freely bind and releasepeptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
...
s. However, the presence of a peptide in the binding domain stimulates the ATPase activity of Hsp70, increasing its normally slow rate of ATP hydrolysis. When ATP is hydrolyzed to ADP the binding pocket of Hsp70 closes, tightly binding the now-trapped peptide chain. Further speeding ATP hydrolysis
ATP hydrolysis is the catabolic reaction process by which chemical energy that has been stored in the high-energy phosphoanhydride bonds in adenosine triphosphate (ATP) is released after splitting these bonds, for example in muscles, by prod ...
are the so-called J-domain cochaperones: primarily Hsp40 in eukaryotes, and DnaJ in prokaryotes. These cochaperones dramatically increase the ATPase activity of Hsp70 in the presence of interacting peptides.
 By binding tightly to partially synthesized peptide sequences (incomplete proteins), Hsp70 prevents them from aggregating and being rendered nonfunctional. Once the entire protein is synthesized, a nucleotide exchange factor (prokaryotic
By binding tightly to partially synthesized peptide sequences (incomplete proteins), Hsp70 prevents them from aggregating and being rendered nonfunctional. Once the entire protein is synthesized, a nucleotide exchange factor (prokaryotic GrpE GrpE (''Gro-P'' like protein E) is a bacterial nucleotide exchange factor that is important for regulation of protein folding machinery, as well as the heat shock response. It is a heat-inducible protein and during stress it prevents unfolded pro ...
, eukaryotic BAG1
BAG family molecular chaperone regulator 1 is a protein that in humans is encoded by the ''BAG1'' gene.
Function
The oncogene BCL2 is a membrane protein that blocks a step in a pathway leading to apoptosis or programmed cell death. The protei ...
and HspBP1 are among those which have been identified) stimulates the release of ADP and binding of fresh ATP, opening the binding pocket. The protein is then free to fold on its own, or to be transferred to other chaperones for further processing. HOP (the Hsp70/Hsp90 Organizing Protein) can bind to both Hsp70 and Hsp90 at the same time, and mediates the transfer of peptides from Hsp70 to Hsp90.
Hsp70 also aids in transmembrane transport of proteins, by stabilizing them in a partially folded state. It is also known to be phosphorylated which regulates several of its functions.
Hsp70 proteins can act to protect cells from thermal or oxidative stress. These stresses normally act to damage proteins, causing partial unfolding and possible aggregation. By temporarily binding to hydrophobic residues exposed by stress, Hsp70 prevents these partially denatured proteins from aggregating, and inhibits them from refolding. Low ATP is characteristic of heat shock and sustained binding is seen as aggregation suppression, while recovery from heat shock involves substrate binding and nucleotide cycling. In a thermophile anaerobe (''Thermotoga maritima'') the Hsp70 demonstrates redox sensitive binding to model peptides, suggesting a second mode of binding regulation based on oxidative stress.
Hsp70 seems to be able to participate in disposal of damaged or defective proteins. Interaction with CHIP Chromatin immunoprecipitation (ChIP) is a type of immunoprecipitation experimental technique used to investigate the interaction between proteins and DNA in the cell. It aims to determine whether specific proteins are associated with specific genom ...
(''C''arboxyl-terminus of ''H''sp70 ''I''nteracting ''P''rotein)–an E3 ubiquitin ligase
A ubiquitin ligase (also called an E3 ubiquitin ligase) is a protein that recruits an E2 ubiquitin-conjugating enzyme that has been loaded with ubiquitin, recognizes a protein substrate, and assists or directly catalyzes the transfer of ubiquit ...
–allows Hsp70 to pass proteins to the cell's ubiquitin
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Fo ...
ation and proteolysis pathways.
Finally, in addition to improving overall protein integrity, Hsp70 directly inhibits apoptosis. One hallmark of apoptosis is the release of cytochrome c, which then recruits Apaf-1 and dATP/ATP into an apoptosome
The apoptosome is a large quaternary protein structure formed in the process of apoptosis. Its formation is triggered by the release of cytochrome c from the mitochondria in response to an internal (intrinsic) or external (extrinsic) cell death st ...
complex. This complex then cleaves procaspase-9, activating caspase-9 and eventually inducing apoptosis via caspase 3
Caspase-3 is a caspase protein that interacts with caspase-8 and caspase-9. It is encoded by the ''CASP3'' gene. ''CASP3'' orthologs have been identified in numerous mammals for which complete genome data are available. Unique orthologs are als ...
activation. Hsp70 inhibits this process by blocking the recruitment of procaspase-9 to the Apaf-1/dATP/cytochrome c apoptosome complex. It does not bind directly to the procaspase-9 binding site, but likely induces a conformational change that renders procaspase-9 binding less favorable. Hsp70 is shown to interact with Endoplasmic reticulum stress sensor protein IRE1alpha thereby protecting the cells from ER stress - induced apoptosis. This interaction prolonged the splicing of XBP-1 mRNA thereby inducing transcriptional upregulation of targets of spliced XBP-1 like EDEM1, ERdj4 and P58IPK rescuing the cells from apoptosis. Other studies suggest that Hsp70 may play an anti-apoptotic role at other steps, but is not involved in Fas-ligand-mediated apoptosis (although Hsp 27 is). Therefore, Hsp70 not only saves important components of the cell (the proteins) but also directly saves the cell as a whole. Considering that stress-response proteins (like Hsp70) evolved before apoptotic machinery, Hsp70's direct role in inhibiting apoptosis provides an interesting evolutionary picture of how more recent (apoptotic) machinery accommodated previous machinery (Hsps), thus aligning the improved integrity of a cell's proteins with the improved chances of that particular cell's survival.
Cancer
Hsp70 is overexpressed inmalignant melanoma
Melanoma, also redundantly known as malignant melanoma, is a type of skin cancer that develops from the pigment-producing cells known as melanocytes. Melanomas typically occur in the skin, but may rarely occur in the mouth, intestines, or eye ...
and underexpressed in renal cell cancer
Renal cell carcinoma (RCC) is a kidney cancer that originates in the lining of the proximal convoluted tubule, a part of the very small tubes in the kidney that transport primary urine. RCC is the most common type of kidney cancer in adults, res ...
.
In breast cancer
Breast cancer is cancer that develops from breast tissue. Signs of breast cancer may include a lump in the breast, a change in breast shape, dimpling of the skin, milk rejection, fluid coming from the nipple, a newly inverted nipple, or a r ...
cell line (MCF7) has been found that not only Hsp90 interacted with estrogen receptor alpha
Estrogen receptor alpha (ERα), also known as NR3A1 (nuclear receptor subfamily 3, group A, member 1), is one of two main types of estrogen receptor, a nuclear receptor (mainly found as a chromatin-binding protein)
that is activated by the sex ...
(ERα) but also Hsp70-1 and Hsc70
Heat shock 70 kDa protein 8 also known as heat shock cognate 71 kDa protein or Hsc70 or Hsp73 is a heat shock protein that in humans is encoded by the ''HSPA8'' gene on chromosome 11. As a member of the heat shock protein 70 family and a chaperon ...
interacted with ERα too.
Given the role of heat shock proteins as an ancient defense system for stabilizing cells and eliminating old and damaged cells, this system has been co-opted by cancer cells to promote their growth. Increased Hsp70 in particular has been shown to inhibit apoptosis of cancer cells, and increased Hsp70 has been shown to be associated with or directly induce endometrial, lung, colon, prostate, and breast cancer, as well as leukemia. Hsp70 in cancer cells may be responsible for tumorigenesis and tumor progression by providing resistance to chemotherapy. Inhibition of Hsp70 has been shown to reduce the size of tumors and can cause their complete regression. Hsp70/Hsp90 is a particularly attractive target for therapeutics, because it is regulated by the inhibition of its ATPase activity, while other HSPs are regulated by nucleotides. Several inhibitors have been designed for Hsp70 that are currently in clinical trials, though as of now HSP90 inhibitors have been more successful. In addition, Hsp70 has been shown to be a regulator of the immune system, activating the immune system as an antigen. Thus, tumor-derived Hsp70 has been suggested as a potential vaccine or avenue to target for immunotherapy. Given the increased expression of Hsp70 in cancer, it has been suggested as a biomarker for cancer prognostics, with high levels portending poor prognosis. An oncogenic mechanism illustrates how extracellular vesicles expressing HSP70 are produced by proliferative Acute Lymphoblastic Leukemia cells and can target and compromise a healthy hematopoiesis system during leukemia development.
Expression in skin tissue
Both Hsp70 andHSP47
Heat shock protein 47, also known as SERPINH1 is a serpin which serves as a human chaperone protein for collagen.
Function
This protein is a member of the serpin superfamily of serine proteinase inhibitors. Its expression is induced by heat sho ...
were shown to be expressed in dermis and epidermis following laser irradiation, and the spatial and temporal changes in HSP expression patterns define the laser-induced thermal damage zone and the process of healing in tissues. Hsp70 may define biochemically the thermal damage zone in which cells are targeted for destruction, and HSP47 may illustrate the process of recovery from thermally induced damage. HSP70 helps in protecting skin against the increased melanin and wrinkled formation induced due to UV exposure.
Neurodegeneration
Inhibition of Hsp90 leads to Hsp70 and Hsp40 upregulation, which can channel misfolded protein for proteasome degradation, which can potentially inhibit the progression of neurodegenerative diseases. For example, Hsp70 overexpression in human neuroglioma cells transfected with mutant alpha-synuclein led to 50% less oligomeric alpha-synuclein species, pointing towards the possibility that increasing its expression could diminish the spread of Parkinson’s disease. Similarly, Hsp70 overexpression suppressed poly-Q dependent aggregation and neurodegeneration in cell cultures, yeast, fly, and mouse models, and deletion of hsp70 increased the size of polyQ inclusion bodies, suggesting that increasing its expression could help to prevent Huntington’s disease. Similarly, reductions in Hsp70 have been shown in transgenic mouse models of ALS and patients with sporadic ALS. Lastly, increased expression or activity of Hsp70 has been proposed as a method to prevent the progression of Alzheimer’s disease, because knock down of Hsp70 promoted A-beta toxicity, and Hsp70 was shown to promote tau stability, while Hsp70 levels are decreased in tauopathies like Alzheimer’s disease. Given the complex interplay between the different chaperone proteins, therapeutic development in this field is aimed at investigating how the chaperone network as a whole can be manipulated and the effect of this manipulation on the progression of neurodegenerative disease, but the balance of Hsp70 and Hsp90 levels appears to be central in this pathophysiology.In diabetes
The fluctuations in the levels of chaperone HSP70 affect the homeostasis. Diabetes leads to several microvasculature and microvasculature diseases like retinopathy, Toll like receptors are integral part of innate immune system and eHSP70 binds to toll like receptors and activates the MyD88 pathway, further stimulating NF-kB, cytokines like TNFα and IL1 β, increased production of reactive oxygen species contributing to insulin resistance and diabetes. Whereas there is decrease in the levels of iHSP70.In cardiovascular diseases
HSP70 is a chaperone with ubiquitous presence. It is crucial in the cardiovascular system. HSP70 normally aids in protein folding and aggregation; when present in the cell, functioning as an anti-inflammatory molecule; however, under stress conditions, it is localized to the extracellular milieu, where it is involved in inducing inflammatory pathways and contributes to disease pathogenesis. It is well established that intracellular HSP70 (iHSP70) levels play a protective role, whereas extracellular HSP70 (eHSP70) levels in circulating blood are linked to pathophysiology in micro and microvasculature, which results in a variety of cardiovascular illnesses. HSP70 homologues identified in human cytosol includes HSPA1A, HSPA1B, HSPA1L, HSPA12B, HSPA13, HSPA14 whereas HSPA9 in mitochondria. The HSP70 acts as DAMP and activates innate immune response which as involved in cardiovascular disease progression. The chaperone protein acts as auto antigen in atherosclerosis. Increased oxidative stress causes the formation of high-density oxidized LDL, the first event in the formation of plaque. This activates HSP70 and its promoter in the endothelial and smooth muscle cells, which contributes to atherosclerosis by inducing JAK/STAT pathway expression. Elevated levels of HSP70 in the serum were largely associated with increased calcification in patients with chronic heart failure, peripheral and renal vascular diseases. HSP70 is also linked to high blood pressure, a worldwide concern and risk factor for a variety of cardiovascular diseases. Hypertension causes endothelial dysfunction and vascular wall damage, both of which contribute to arterial stiffness and atherosclerosis. HSPA1A, HSPA1B, and HSPA1L are three genes in humans that encode HSP70, and their polymorphism is linked to the onset of high blood pressure and cardiovascular disease. Angiotensin II, endothelin-1, or phenylepinephrine cause HSP70 overexpression, which activates several molecular pathways, resulting in increased production of ROS, CRP, IL-10, TNF-alpha, and IL-6 These inflammatory signals interfere with the antioxidant machinery and results in rapid disease progression. HSP70 expression increases after the coronary bypass surgery. Exercise has a positive and protective impact on cardiovascular disorders and stimulates the increased production of chaperone protein together known to be cardioprotective.Family members
Prokaryotes express three Hsp70 proteins: DnaK, HscA (Hsc66), and HscC (Hsc62). Eukaryotic organisms express several slightly different Hsp70 proteins. All share the common domain structure, but each has a unique pattern of expression or subcellular localization. These are, among others: *Hsc70
Heat shock 70 kDa protein 8 also known as heat shock cognate 71 kDa protein or Hsc70 or Hsp73 is a heat shock protein that in humans is encoded by the ''HSPA8'' gene on chromosome 11. As a member of the heat shock protein 70 family and a chaperon ...
(Hsp73/HSPA8) is a constitutively expressed chaperone protein. It typically makes up one to three percent of total cellular protein.
* Hsp70 (encoded by three very closely related paralogs: HSPA1A
Heat shock 70 kDa protein 1, also termed Hsp72, is a protein that in humans is encoded by the ''HSPA1A'' gene. As a member of the heat shock protein 70 family and a chaperone protein, it facilitates the proper folding of newly translated and misfo ...
, HSPA1B, and HSPA1L) is a stress-induced protein. High levels can be produced by cells in response to hyperthermia, oxidative stress, and changes in pH.
* Binding immunoglobulin protein
Binding immunoglobulin protein (BiP) also known as 78 kDa glucose-regulated protein (GRP-78) or heat shock 70 kDa protein 5 (HSPA5) is a protein that in humans is encoded by the ''HSPA5'' gene.
BiP is a HSP70 molecular chaperone located in the l ...
(BiP or Grp78) is a protein localized to the endoplasmic reticulum. It is involved in protein folding there, and can be upregulated in response to stress or starvation.
* mtHsp70 or Grp75 is the mitochondrial Hsp70.
The following is a list of human Hsp70 genes and their corresponding proteins:
Hsps 90 and 110
 Hsp90s are essential for protein remodeling, similar to Hsp70 proteins, and play an especially vital role in eukaryotes, where it has been suggested that Hsp90 interacts with the DnaK system (composed of DnaK, GrpE, and either DnaJ or CbpA) to facilitate the process of protein remodeling. In E. coli, Hsp90s works collaboratively with Hsp70s to facilitate protein remodeling and activation. Hsp90Ec and DnaK are chaperones of Hsp90 and Hsp70, respectively. DnaK initially binds and stabilizes the misfolded protein before working collaboratively with Hsp90Ec to refold this substrate and cause its activation. Given conditions of excess DnaK, this chaperone has been found to inhibit remodeling of proteins. However, the presence of Hsp90Ec can mitigate this effect and enable protein remodeling despite conditions of excess DnaK.
The Hsp70 superfamily also includes a family of Hsp110/Grp170 (Sse) proteins, which are larger proteins related to Hsp70. The Hsp110 family of proteins have divergent functions: yeast Sse1p has little ATPase activity but is a chaperone on its own as well as a nucleotide exchange factor for Hsp70, while the closely related Sse2p has little unfoldase activity.
The following is a list of currently named human HSP110 genes. HSPH2-4 are proposed names and the current name is linked:
Hsp90s are essential for protein remodeling, similar to Hsp70 proteins, and play an especially vital role in eukaryotes, where it has been suggested that Hsp90 interacts with the DnaK system (composed of DnaK, GrpE, and either DnaJ or CbpA) to facilitate the process of protein remodeling. In E. coli, Hsp90s works collaboratively with Hsp70s to facilitate protein remodeling and activation. Hsp90Ec and DnaK are chaperones of Hsp90 and Hsp70, respectively. DnaK initially binds and stabilizes the misfolded protein before working collaboratively with Hsp90Ec to refold this substrate and cause its activation. Given conditions of excess DnaK, this chaperone has been found to inhibit remodeling of proteins. However, the presence of Hsp90Ec can mitigate this effect and enable protein remodeling despite conditions of excess DnaK.
The Hsp70 superfamily also includes a family of Hsp110/Grp170 (Sse) proteins, which are larger proteins related to Hsp70. The Hsp110 family of proteins have divergent functions: yeast Sse1p has little ATPase activity but is a chaperone on its own as well as a nucleotide exchange factor for Hsp70, while the closely related Sse2p has little unfoldase activity.
The following is a list of currently named human HSP110 genes. HSPH2-4 are proposed names and the current name is linked:
See also
*Heat shock protein 70 (Hsp70) internal ribosome entry site (IRES)
The heat shock protein 70 (Hsp70) internal ribosome entry site (IRES) is an RNA element that allows cap independent translation during conditions such as heat shock and stress. It has been shown that the 216 nucleotide
Nucleotides are organ ...
References
External links * {{Chaperones Heat shock proteins Molecular chaperones