|
C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, carboxy tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ... or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Targeting
Protein targeting or protein sorting is the biological mechanism by which proteins are transported to their appropriate destinations within or outside the cell. Proteins can be targeted to the inner space of an organelle, different intracellular membranes, the plasma membrane, or to the exterior of the cell via secretion. Information contained in the protein itself directs this delivery process. Correct sorting is crucial for the cell; errors or dysfunction in sorting have been linked to multiple diseases. History In 1970, Günter Blobel conducted experiments on protein translocation across membranes. Blobel, then an assistant professor at Rockefeller University, built upon the work of his colleague George Palade. Palade had previously demonstrated that non-secreted proteins were translated by free ribosomes in the cytosol, while secreted proteins (and target proteins, in general) were translated by ribosomes bound to the endoplasmic reticulum (ER). Candidate explanations at t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prenylation
Prenylation (also known as isoprenylation or lipidation) is the addition of hydrophobic molecules to a protein or a biomolecule. It is usually assumed that prenyl groups (3-methylbut-2-en-1-yl) facilitate attachment to cell membranes, similar to lipid anchors like the GPI anchor, though direct evidence of this has not been observed. Prenyl groups (also called isoprenyl groups, having one hydrogen atom more than isoprene) have been shown to be important for protein–protein binding through specialized prenyl-binding domains. Protein prenylation Protein prenylation involves the transfer of either a farnesyl or a geranylgeranyl moiety to C-terminal cysteine(s) of the target protein. There are three enzymes that carry out prenylation in the cell, farnesyl transferase, Caax protease and geranylgeranyl transferase I. Farnesylation is a type of prenylation, a post-translational modification of proteins by which an isoprenyl group is added to a cysteine residue. It is an important ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polypeptide
Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides that have a molecular mass of 10,000 Da or more are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. Peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyclic peptides have an N-terminal (amine group) and C-terminal (ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Posttranslational Modification
In molecular biology, post-translational modification (PTM) is the covalent process of changing proteins following protein biosynthesis. PTMs may involve enzymes or occur spontaneously. Proteins are created by ribosomes, which translate mRNA into polypeptide chains, which may then change to form the mature protein product. PTMs are important components in cell signalling, as for example when prohormones are converted to hormones. Post-translational modifications can occur on the amino acid side chains or at the protein's C- or N- termini. They can expand the chemical set of the 22 amino acids by changing an existing functional group or adding a new one such as phosphate. Phosphorylation is highly effective for controlling the enzyme activity and is the most common change after translation. Many eukaryotic and prokaryotic proteins also have carbohydrate molecules attached to them in a process called glycosylation, which can promote protein folding and improve stability a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrapeptide Structural Formulae V
A tetrapeptide is a peptide, classified as an oligopeptide, since it only consists of four amino acids joined by peptide bonds. Many tetrapeptides are pharmacologically active, often showing affinity and specificity for a variety of receptors in protein-protein signaling. Present in nature are both linear and cyclic tetrapeptides (CTPs), the latter of which mimics protein reverse turns which are often present on the surface of proteins and druggable targets. Tetrapeptides may be cyclized by a fourth peptide bond or other covalent bonds. Examples of tetrapeptides are: * Tuftsin (L-threonyl-L-lysyl-L-prolyl-L-arginine) is a peptide related primarily to the immune system function. * Rigin (glycyl-L-glutaminyl-L-prolyl-L-arginine) is a tetrapeptide with functions similar to those of tuftsin. * Postin (Lys-Pro-Pro-Arg) is the N-terminal tetrapeptide of cystatin C and an antagonist of tuftsin. * Endomorphin-1 (H-Tyr-Pro-Trp-Phe-NH2) and endomorphin-2 (H-Tyr-Pro-Phe-Phe-NH2) are pe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
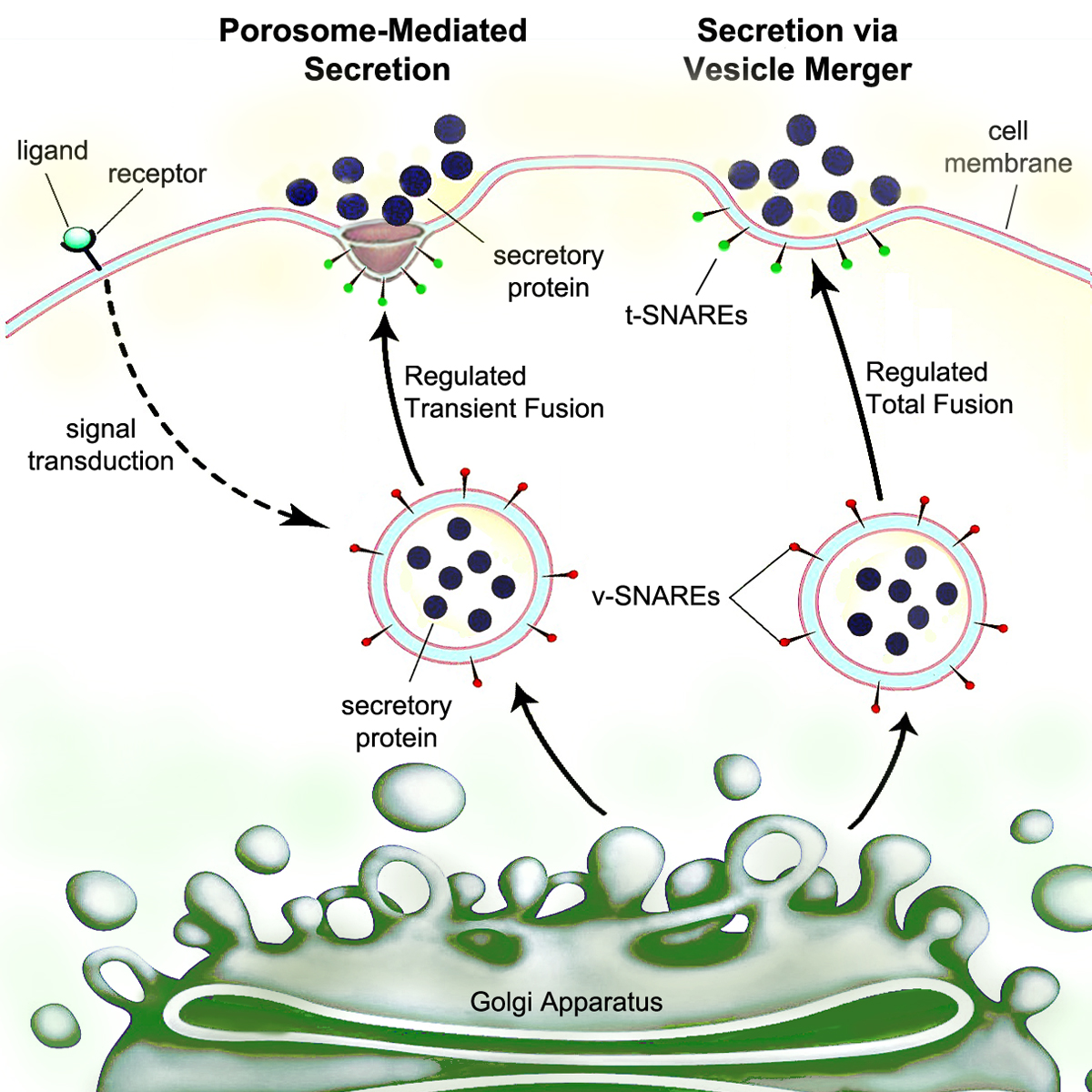
Secretory Pathway
Secretion is the movement of material from one point to another, such as a secreted chemical substance from a cell (biology), cell or gland. In contrast, excretion is the removal of certain substances or waste products from a cell or organism. The classical mechanism of cell secretion is via secretory portals at the Cell membrane, plasma membrane called porosomes. Porosomes are permanent cup-shaped lipoprotein structures embedded in the cell membrane, where secretory vesicles transiently dock and fuse to release intra-vesicular contents from the cell. Bacterial secretion system, Secretion in bacterial species means the transport or translocation of effector molecules. For example: proteins, enzymes or toxins (such as cholera toxin in pathogenic bacteria e.g. ''Vibrio cholerae'') from across the interior (cytoplasm or cytosol) of a bacterial cell to its exterior. Secretion is a very important mechanism in bacterial functioning and operation in their natural surrounding environment ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
G Protein
G proteins, also known as guanine nucleotide-binding proteins, are a Protein family, family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell (biology), cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases. There are two classes of G proteins. The first function as monomeric small GTPases (small G-proteins), while the second function as heterotrimeric G protein protein complex, complexes. The latter class of complexes is made up of ''G alpha subunit, alpha'' (Gα), ''beta'' (Gβ) and ''gamma'' (Gγ) protein subunit, subunits. In addition, the beta and gamma subunits can form a stable Protein dimer, dimeric complex re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxisome
A peroxisome () is a membrane-bound organelle, a type of microbody, found in the cytoplasm of virtually all eukaryotic cells. Peroxisomes are oxidative organelles. Frequently, molecular oxygen serves as a co-substrate, from which hydrogen peroxide (H2O2) is then formed. Peroxisomes owe their name to hydrogen peroxide-generating and scavenging activities. They perform key roles in lipid metabolism and the redox, reduction of reactive oxygen species. Peroxisomes are involved in the catabolism of very long chain fatty acids, branched chain fatty acids, bile acid intermediates (in the liver), D-amino acids, and polyamines. Peroxisomes also play a role in the biosynthesis of plasmalogens: ether phospholipids critical for the normal function of mammalian brains and lungs. Peroxisomes contain approximately 10% of the total activity of two enzymes (Glucose-6-phosphate dehydrogenase and Phosphogluconate dehydrogenase, 6-Phosphogluconate dehydrogenase) in the pentose phosphate pathway, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosylphosphatidylinositol
Glycosylphosphatidylinositol () or glycophosphatidylinositol (GPI) is a phosphoglyceride that can be attached to the C-terminus of a protein during posttranslational modification. The resulting GPI-anchored proteins play key roles in a wide variety of biological processes. GPI is composed of a phosphatidylinositol group linked through a carbohydrate-containing linker (glucosamine and mannose glycosidically bound to the inositol residue) and via an ethanolamine phosphate (EtNP) bridge to the C-terminal amino acid of a mature protein. The two fatty acids within the hydrophobic phosphatidyl-inositol group anchor the protein to the cell membrane. Synthesis Glycosylated (GPI-anchored) proteins contain a signal sequence, thus directing them to the endoplasmic reticulum (ER). The protein is co-translationally inserted in the ER membrane via a translocon and is attached to the ER membrane by its hydrophobic C terminus; the majority of the protein extends into the ER lumen. The hy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |