Felkin Model on:
[Wikipedia]
[Google]
[Amazon]
In stereochemistry, asymmetric induction (also enantioinduction) describes the preferential formation in a


 Various chiral ligands have been developed to prepare chiral allylmetals for the reaction with aldehydes. H. C. Brown was the first to report the chiral allylboron reagents for asymmetric allylation reactions with aldehydes. The chiral allylboron reagents were synthesized from the natural product (+)-a-pinene in two steps. The TADDOL ligands developed by
Various chiral ligands have been developed to prepare chiral allylmetals for the reaction with aldehydes. H. C. Brown was the first to report the chiral allylboron reagents for asymmetric allylation reactions with aldehydes. The chiral allylboron reagents were synthesized from the natural product (+)-a-pinene in two steps. The TADDOL ligands developed by 
The Evolution of Models for Carbonyl Addition
Evans Group Afternoon Seminar Sarah Siska February 9, 2001 {{DEFAULTSORT:Asymmetric Induction Stereochemistry Induct
chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
of one enantiomer or diastereoisomer
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have dif ...
over the other as a result of the influence of a chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from i ...
feature present in the substrate, reagent, catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
or environment. Asymmetric induction is a key element in asymmetric synthesis.
Asymmetric induction was introduced by Hermann Emil Fischer
Hermann Emil Louis Fischer (; 9 October 1852 – 15 July 1919) was a German chemist and 1902 recipient of the Nobel Prize in Chemistry. He discovered the Fischer esterification. He also developed the Fischer projection, a symbolic way of dra ...
based on his work on carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or m ...
s. Several types of induction exist.
Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis The chiral pool is a "collection of abundant enantiopure building blocks provided by nature" used in synthesis. In other words, a chiral pool would be a large quantity of common organic enantiomers. Contributors to the chiral pool are amino acids, s ...
. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked ...
through a catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
of chiral ligand. This method of asymmetric synthesis is economically most desirable.
Carbonyl 1,2 asymmetric induction
Several models exist to describe chiral induction at carbonyl carbons during nucleophilic additions. These models are based on a combination of steric and electronic considerations and are often in conflict with each other. Models have been devised by Cram (1952), Cornforth (1959), Felkin (1969) and others.Cram's rule
The Cram's rule of asymmetric induction developed by Donald J. Cram in 1952 is an early concept relating to the prediction of stereochemistry in certain acyclic systems. In full the rule is: ''In certain non-catalytic reactions that diastereomer will predominate, which could be formed by the approach of the entering group from the least hindered side when the rotational conformation of the C-C bond is such that the double bond is flanked by the two least bulky groups attached to the adjacent asymmetric center.'' The rule indicates that the presence of an asymmetric center in a molecule induces the formation of an asymmetric center adjacent to it based onsteric hindrance
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
.
In his 1952 publication Cram presented a large number of reactions described in the literature for which the conformation of the reaction products could be explained based on this rule and he also described an elaborate experiment (''scheme 1'') making his case.
:
The experiments involved two reactions. In experiment one ''2-phenylpropionaldehyde'' (1, racemic
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. ...
but (R)-enantiomer shown) was reacted with the Grignard reagent of bromobenzene
Bromobenzene is an aryl halide, C6H5Br. It is a colourless liquid although older samples can appear yellow. It is a reagent in organic synthesis.
Synthesis and reactions
Bromobenzene is prepared by the action of bromine on benzene in the presenc ...
to ''1,2-diphenyl-1-propanol'' (2) as a mixture of diastereomer
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have di ...
s, predominantly the threo
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have di ...
isomer (see for explanation the Fischer projection
In chemistry, the Fischer projection, devised by Emil Fischer in 1891, is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer projections were originally proposed for the depiction of carbohydrates ...
).
The preference for the formation of the threo isomer can be explained by the rule stated above by having the active nucleophile in this reaction attacking the carbonyl group
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
from the least hindered side (see Newman projection
A Newman projection is a drawing that helps visualize the 3-dimensional structure of a molecule. This projection most commonly sights down a carbon-carbon bond, making it a very useful way to visualize the stereochemistry of alkanes. A Newman pro ...
A) when the carbonyl is positioned in a staggered formation with the methyl group and the hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
atom, which are the two smallest substituents creating a minimum of steric hindrance
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
, in a gauche orientation and phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen ...
as the most bulky group in the anti conformation.
The second reaction is the organic reduction
Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions, because many reactions car ...
of ''1,2-diphenyl-1-propanone'' 2 with lithium aluminium hydride, which results in the same reaction product as above but now with preference for the erythro
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have dif ...
isomer (2a). Now a hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride ...
anion (H−) is the nucleophile attacking from the least hindered side (imagine hydrogen entering from the paper plane).
In the original 1952 publication, additional evidence was obtained for the structural assignment of the reaction products by applying them to a Chugaev elimination
The Chugaev elimination is a chemical reaction that involves the elimination of water from alcohols to produce alkenes. The intermediate is a xanthate. It is named for its discoverer, the Russian chemist Lev Aleksandrovich Chugaev (1873-1922), who ...
, wherein the threo isomer reacts to the cis isomer
Cis or cis- may refer to:
Places
* Cis, Trentino, in Italy
* In Poland:
** Cis, Świętokrzyskie Voivodeship, south-central
** Cis, Warmian-Masurian Voivodeship, north
Math, science and biology
* cis (mathematics) (cis(''θ'')), a trigonom ...
of -α-methyl-stilbene Stilbene may refer to one of the two stereoisomers of 1,2-diphenylethene:
* (''E'')-Stilbene (''trans'' isomer)
* (''Z'')-Stilbene (''cis'' isomer)
See also
* Stilbenoid
Stilbenoids are hydroxylated derivatives of stilbene. They have a C6– ...
and the erythro isomer to the trans version.
:
Felkin model
The Felkin model (1968) named after Hugh Felkin also predicts the stereochemistry of nucleophilic addition reactions tocarbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
groups. Felkin argued that the Cram model suffered a major drawback: an eclipsed conformation in the transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked ...
between the carbonyl substituent (the hydrogen atom in aldehydes) and the largest α-carbonyl substituent. He demonstrated that by increasing the steric bulk of the carbonyl substituent from methyl to ethyl to isopropyl
In organic chemistry, propyl is a three-carbon alkyl substituent with chemical formula for the linear form. This substituent form is obtained by removing one hydrogen atom attached to the terminal carbon of propane. A propyl substituent is often ...
to isobutyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane.
The isomer ''n''-butane can connect in two ways, givi ...
, the stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non-stereospecific creation of a new stereocenter or during a non-stereospecific transformation of ...
also increased, which is not predicted by Cram's rule:
:
The Felkin rules are:
* The transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked ...
s are reactant-like.
* Torsional strain (Pitzer strain) involving partial bonds (in transition states) represents a substantial fraction of the strain between fully formed bonds, even when the degree of bonding is quite low. The conformation in the TS is staggered and not eclipsed with the substituent R skew with respect to two adjacent groups one of them the smallest in TS A.
:
: For comparison TS B is the Cram transition state.
* The main steric interactions involve those around R and the nucleophile but not the carbonyl oxygen atom.
* Attack of the nucleophile occurs according to the Dunitz angle (107 degrees), eclipsing the hydrogen, rather than perpendicular to the carbonyl.
* A polar effect
The polar effect or electronic effect in chemistry is the effect exerted by a substituent on modifying electrostatic forces operating on a nearby reaction center. The main contributors to the polar effect are the inductive effect, mesomeric effec ...
or electronic effect stabilizes a transition state with maximum separation between the nucleophile and an electron-withdrawing group
In chemistry, an electron-withdrawing group (EWG) is a substituent that has some of the following kinetic and thermodynamic implications:
*with regards to electron transfer, electron-withdrawing groups enhance the oxidizing power tendency of the ...
. For instance haloketone
In organic chemistry, an α-haloketone is a functional group consisting of a ketone group or more generally a carbonyl group with an α-halogen substituent. α-haloketones are alkylating agents. Prominent α-haloketones include phenacyl bromide ...
s do not obey Cram's rule, and, in the example above, replacing the electron-withdrawing phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen ...
group by a cyclohexyl
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colorless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
group reduces stereoselectivity considerably.
Felkin–Anh model
The Felkin–Anh model is an extension of the Felkin model that incorporates improvements suggested by Nguyễn Trọng Anh and Odile Eisenstein to correct for two key weaknesses in Felkin's model. The first weakness addressed was the statement by Felkin of a strong polar effect in nucleophilic addition transition states, which leads to the complete inversion of stereochemistry by SN2 reactions, without offering justifications as to why this phenomenon was observed. Anh's solution was to offer the antiperiplanar effect as a consequence of asymmetric induction being controlled by both substituent and orbital effects.Anh, N. T.; Eisenstein, ''O. Nouv. J. Chim.'' 1977, ''1'', 61. In this effect, the best nucleophile acceptor σ* orbital is aligned parallel to both the π and π* orbitals of the carbonyl, which provide stabilization of the incoming anion. The second weakness in the Felkin Model was the assumption of substituent minimization around the carbonyl R, which cannot be applied to aldehydes. Incorporation ofBürgi–Dunitz angle
The Bürgi–Dunitz angle (BD angle) is one of two angles that fully define the geometry of "attack" (approach via collision) of a nucleophile on a trigonal unsaturated center in a molecule, originally the carbonyl center in an organic ketone, ...
ideas allowed Anh to postulate a non-perpendicular attack by the nucleophile on the carbonyl center, anywhere from 95° to 105° relative to the oxygen-carbon double bond, favoring approach closer to the smaller substituent and thereby solve the problem of predictability for aldehydes.
Anti–Felkin selectivity
Though the Cram and Felkin–Anh models differ in theconformers
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by rotations about formally single bonds (refer to figure on single bond rotation). While any two arrangements of atoms in a molec ...
considered and other assumptions, they both attempt to explain the same basic phenomenon: the preferential addition of a nucleophile to the most sterically favored face of a carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
moiety. However, many examples exist of reactions that display stereoselectivity opposite of what is predicted by the basic tenets of the Cram and Felkin–Anh models. Although both of the models include attempts to explain these reversals, the products obtained are still referred to as "anti-Felkin" products. One of the most common examples of altered asymmetric induction selectivity requires an α-carbon substituted with a component with Lewis base character (i.e. O, N, S, P substituents). In this situation, if a Lewis acid such as Al-iPr2 or Zn2+ is introduced, a bidentate chelation
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are ...
effect can be observed. This locks the carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
and the Lewis base substituent in an eclipsed conformation, and the nucleophile will then attack from the side with the smallest free α-carbon substituent. If the chelating R group is identified as the largest, this will result in an "anti-Felkin" product.
This stereoselective
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non-stereospecific creation of a new stereocenter or during a non-stereospecific transformation of ...
control was recognized and discussed in the first paper establishing the Cram model, causing Cram to assert that his model requires non-chelating conditions. An example of chelation
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are ...
control of a reaction can be seen here, from a 1987 paper that was the first to directly observe such a "Cram-chelate" intermediate, vindicating the model:
Here, the methyl titanium chloride forms a Cram-chelate. The methyl group then dissociates from titanium
Titanium is a chemical element with the Symbol (chemistry), symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resista ...
and attacks the carbonyl, leading to the anti-Felkin diastereomer.
A non-chelating electron-withdrawing substituent effect can also result in anti-Felkin selectivity. If a substituent on the α-carbon is sufficiently electron withdrawing, the nucleophile will add ''anti-'' relative to the electron withdrawing group
In chemistry, an electron-withdrawing group (EWG) is a substituent that has some of the following kinetic and thermodynamic implications:
*with regards to electron transfer, electron-withdrawing groups enhance the oxidizing power tendency of ...
, even if the substituent is not the largest of the 3 bonded to the α-carbon. Each model offers a slightly different explanation for this phenomenon. A polar effect was postulated by the Cornforth model and the original Felkin model, which placed the EWG substituent and incoming nucleophile ''anti''- to each other in order to most effectively cancel the dipole moment of the transition structure.
This Newman projection
A Newman projection is a drawing that helps visualize the 3-dimensional structure of a molecule. This projection most commonly sights down a carbon-carbon bond, making it a very useful way to visualize the stereochemistry of alkanes. A Newman pro ...
illustrates the Cornforth and Felkin transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked ...
that places the EWG ''anti-'' to the incoming nucleophile, regardless of its steric bulk relative to RS and RL.
The improved Felkin–Anh model, as discussed above, makes a more sophisticated assessment of the polar effect by considering molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of findin ...
interactions in the stabilization of the preferred transition state. A typical reaction illustrating the potential anti-Felkin selectivity of this effect, along with its proposed transition structure, is pictured below:
Carbonyl 1,3 asymmetric induction
It has been observed that the stereoelectronic environment at the β-carbon of can also direct asymmetric induction. A number of predictive models have evolved over the years to define the stereoselectivity of such reactions.Chelation model
According to Reetz, the Cram-chelate model for 1,2-inductions can be extended to predict the chelated complex of a β-alkoxy aldehyde and metal. The nucleophile is seen to attack from the less sterically hindered side and ''anti-'' to the substituent Rβ, leading to the ''anti-''adduct as the major product. To make such chelates, the metal center must have at least two free coordination sites and the protecting ligands should form a bidentate complex with the Lewis acid.Non-chelation model
Cram–Reetz model
Cram and Reetz demonstrated that 1,3-stereocontrol is possible if the reaction proceeds through an acyclic transition state. The reaction of β-alkoxy aldehyde with allyltrimethylsilane showed good selectivity for the ''anti-''1,3-diol, which was explained by the Cram polar model. The polar benzyloxy group is oriented anti to the carbonyl to minimize dipole interactions and the nucleophile attacks ''anti-'' to the bulkier (RM) of the remaining two substituents.Evans model
More recently, Evans presented a different model for nonchelate 1,3-inductions. In the proposed transition state, the β-stereocenter is oriented ''anti-'' to the incoming nucleophile, as seen in the Felkin–Anh model. The polar X group at the β-stereocenter is placed ''anti-'' to the carbonyl to reduce dipole interactions, and Rβ is placed ''anti-'' to the aldehyde group to minimize the steric hindrance. Consequently, the 1,3-''anti''-diol would be predicted as the major product.Carbonyl 1,2 and 1,3 asymmetric induction
If the substrate has both an α- and β-stereocenter, the Felkin–Anh rule (1,2-induction) and the Evans model (1,3-induction) should considered at the same time. If these two stereocenters have an ''anti-'' relationship, both models predict the same diastereomer (the stereoreinforcing case). However, in the case of the syn-substrate, the Felkin–Anh and the Evans model predict different products (non-stereoreinforcing case). It has been found that the size of the incoming nucleophile determines the type of control exerted over the stereochemistry. In the case of a large nucleophile, the interaction of the α-stereocenter with the incoming nucleophile becomes dominant; therefore, the Felkin product is major one. Smaller nucleophiles, on the other hand, result in 1,3 control determining the asymmetry.Acyclic alkenes asymmetric induction
Chiral acyclic alkenes also showdiastereoselectivity
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have dif ...
upon reactions such as epoxidation
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale ...
and enolate alkylation. The substituents around the alkene can favour the approach of the electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carrie ...
from one or the other face of the molecule. This is the basis of the Houk's model, based on theoretical work by Kendall Houk, which predicts that the selectivity is stronger for ''cis'' than for ''trans'' double bonds.
:
In the example shown, the ''cis'' alkene assumes the shown conformation to minimize steric clash
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
between RS and the methyl group. The approach of the electrophile preferentially occurs from the same side of the medium group (RM) rather than the large group (RL), mainly producing the shown diastereoisomer. Since for a ''trans'' alkene the steric hindrance between RS and the H group is not as large as for the ''cis'' case, the selectivity is much lower.



Substrate control: asymmetric induction by molecular framework in acyclic systems
Asymmetric induction by the molecular framework of an acyclic substrate is the idea that asymmetric steric andelectronic
Electronic may refer to:
*Electronics, the science of how to control electric energy in semiconductor
* ''Electronics'' (magazine), a defunct American trade journal
*Electronic storage, the storage of data using an electronic device
*Electronic co ...
properties of a molecule may determine the chirality of subsequent chemical reactions on that molecule. This principal is used to design chemical syntheses where one stereocentre is in place and additional stereocentres are required.
When considering how two functional groups
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
or species react, the precise 3D configurations of the chemical entities involved will determine how they may approach one another. Any restrictions as to how these species may approach each other will determine the configuration of the product of the reaction. In the case of asymmetric induction, we are considering the effects of one asymmetric centre on a molecule on the reactivity of other functional groups on that molecule. The closer together these two sites are, the larger an influence is expected to be observed. A more holistic approach to evaluating these factors is by computational modelling
Computer simulation is the process of mathematical modelling, performed on a computer, which is designed to predict the behaviour of, or the outcome of, a real-world or physical system. The reliability of some mathematical models can be deter ...
, however, simple qualitative factors may also be used to explain the predominant trends seen for some synthetic steps. The ease and accuracy of this qualitative approach means it is more commonly applied in synthesis and substrate design. Examples of appropriate molecular frameworks are alpha chiral aldehydes and the use of chiral auxiliaries.
Asymmetric induction at alpha-chiral aldehydes
Possible reactivity at aldehydes includenucleophilic attack
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
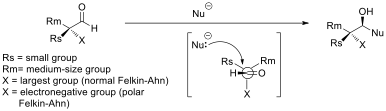
and addition of allylmetals. The stereoselectivity of nucleophilic attack at alpha-chiral aldehydes may be described by the Felkin–Anh or polar Felkin Anh models and addition of achiral allylmetals may be described by Cram’s rule.
Felkin–Anh and polar Felkin–Anh model
Selectivity in nucleophilic additions to chiral aldehydes is often explained by the Felkin–Anh model (see figure). The nucleophile approaches the carbon of the carbonyl group at the Burgi-Dunitz angle. At this trajectory, attack from the bottom face is disfavored due to steric bulk of the adjacent, large, functional group. The polar Felkin–Anh model is applied in the scenario where X is an electronegative group. The polar Felkin–Anh model postulates that the observed stereochemistry arises due to hyperconjugative stabilization arising from the anti-periplanar interaction between the C-X antibonding σ* orbital and the forming bond. Improving Felkin–Anh selectivity for organometal additions to aldehydes can be achieved by using organo-aluminum nucleophiles instead of the corresponding Grignard or organolithium nucleophiles. Claude Spino and co-workers have demonstrated significant stereoselectivity improvements upon switching from vinylgrignard to vinylalane reagents with a number of chiral aldehydes.Cram’s rule
Addition of achiral allylmetals toaldehydes
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
forms a chiral alcohol, the stereochemical outcome of this reaction is determined by the chirality of the α-carbon
In the nomenclature of organic chemistry, a locant is a term to indicate the position of a functional group or substituent within a molecule.
Numeric locants
The International Union of Pure and Applied Chemistry (IUPAC) recommends the use of ...
on the aldehyde substrate (Figure "Substrate control: addition of achiral allylmetals to α-chiral aldehydes"). The allylmetal reagents used include boron, tin
Tin is a chemical element with the symbol Sn (from la, stannum) and atomic number 50. Tin is a silvery-coloured metal.
Tin is soft enough to be cut with little force and a bar of tin can be bent by hand with little effort. When bent, t ...
and titanium
Titanium is a chemical element with the Symbol (chemistry), symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resista ...
.
Cram’s rule explains the stereoselectivity by considering the transition state depicted in figure 3. In the transition state the oxygen lone pair is able to interact with the boron centre whilst the allyl group is able to add to the carbon end of the carbonyl group. The steric demand of this transition state is minimized by the α-carbon configuration holding the largest group away from (trans to) the congested carbonyl group and the allylmetal group approaching past the smallest group on the α-carbon centre. In the example below (Figure "An example of substrate controlled addition of achiral allyl-boron to α-chiral aldehyde"), (R)-2-methylbutanal (1) reacts with the allylboron reagent (2) with two possible diastereomers of which the (R, R)-isomer is the major product. The Cram model of this reaction is shown with the carbonyl group placed trans to the ethyl group (the large group) and the allyl boron approaching past the hydrogen (the small group). The structure is shown in Newman projection
A Newman projection is a drawing that helps visualize the 3-dimensional structure of a molecule. This projection most commonly sights down a carbon-carbon bond, making it a very useful way to visualize the stereochemistry of alkanes. A Newman pro ...
. In this case the nucleophilic addition reaction happens at the face where the hydrogen (the small group) is, producing the (R, R)-isomer as the major product.
Chiral auxiliaries
Asymmetric stereoinduction can be achieved with the use of chiral auxiliaries. Chiral auxiliaries may be reversibly attached to the substrate, inducing a diastereoselective reaction prior to cleavage, overall producing an enantioselective process. Examples of chiral auxiliaries include, Evans’ chiral oxazolidinone auxiliaries (for asymmetric aldol reactions) pseudoephedrine amides andtert-butanesulfinamide
''tert''-Butanesulfinamide (also known as 2-methyl-2-propanesulfinamide or Ellman's sulfinamide) is an organosulfur compound and a member of the class of sulfinamides. Both enantiomeric forms are commercially available and are used in asymmetr ...
imines.
Substrate control: asymmetric induction by molecular framework in cyclic systems
Cyclic molecule
A cyclic compound (or ring compound) is a term for a chemical compound, compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a Ring (chemistry), ring. Rings may vary in size from three to many ...
s often exist in much more rigid conformations than their linear counterparts. Even very large macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry.
...
s like erythromycin exist in defined geometries despite having many degrees of freedom. Because of these properties, it is often easier to achieve asymmetric induction with macrocyclic substrates rather than linear ones. Early experiments performed by W. Clark Still and colleagues showed that medium- and large-ring organic molecules can provide striking levels of stereo induction as substrates in reactions such as kinetic enolate
In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl () compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds.
Bonding and structure
Enolate anions are electr ...
alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecti ...
, dimethylcuprate addition, and catalytic hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organ ...
. Even a single methyl group is often sufficient to bias the diastereomeric outcome of the reaction. These studies, among others, helped challenge the widely-held scientific belief that large rings are too floppy to provide any kind of stereochemical control.
A number of total syntheses have made use of macrocyclic stereocontrol
Macrocyclic stereocontrol refers to the directed outcome of a given intermolecular or intramolecular chemical reaction, generally an organic reaction, that is governed by the conformational or geometrical preference of a carbocyclic or heterocyc ...
to achieve desired reaction products. In the synthesis of (−)-cladiella-6,11-dien-3-ol, a strained trisubstituted olefin was dihydroxylated diasetereoselectively with ''N''-methylmorpholine ''N''-oxide (NMO) and osmium tetroxide
Osmium tetroxide (also osmium(VIII) oxide) is the chemical compound with the formula OsO4. The compound is noteworthy for its many uses, despite its toxicity and the rarity of osmium. It also has a number of unusual properties, one being that the ...
, in the presence of an unstrained olefin. En route to (±)-periplanone B, chemists achieved a facial selective epoxidation of an enone intermediate using tert-butyl hydroperoxide in the presence of two other alkenes. Sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula Na BH4. This white solid, usually encountered as an aqueous basic solution, is a reducing agent that finds applica ...
reduction of a 10-membered ring enone intermediate en route to the sesquiterpene
Sesquiterpenes are a class of terpenes that consist of three isoprene units and often have the molecular formula C15H24. Like monoterpenes, sesquiterpenes may be cyclic or contain rings, including many unique combinations. Biochemical modificat ...
eucannabinolide proceeded as predicted by molecular modelling calculations that accounted for the lowest energy macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry.
...
conformation. Substrate-controlled synthetic schemes have many advantages, since they do not require the use of complex asymmetric reagents to achieve selective transformations.
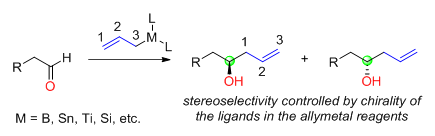
Reagent control: addition of chiral allylmetals to achiral aldehydes
In organic synthesis, reagent control is an approach to selectively forming onestereoisomer
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in ...
out of many, the stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non-stereospecific creation of a new stereocenter or during a non-stereospecific transformation of ...
is determined by the structure and chirality of the reagent used. When chiral allylmetals are used for nucleophilic addition reaction to achiral aldehydes
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
, the chirality of the newly generated alcohol carbon is determined by the chirality of the allymetal reagents (Figure 1). The chirality of the allymetals usually comes from the asymmetric ligands used. The metals in the allylmetal reagents include boron, tin
Tin is a chemical element with the symbol Sn (from la, stannum) and atomic number 50. Tin is a silvery-coloured metal.
Tin is soft enough to be cut with little force and a bar of tin can be bent by hand with little effort. When bent, t ...
, titanium
Titanium is a chemical element with the Symbol (chemistry), symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resista ...
, silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ta ...
, etc.
 Various chiral ligands have been developed to prepare chiral allylmetals for the reaction with aldehydes. H. C. Brown was the first to report the chiral allylboron reagents for asymmetric allylation reactions with aldehydes. The chiral allylboron reagents were synthesized from the natural product (+)-a-pinene in two steps. The TADDOL ligands developed by
Various chiral ligands have been developed to prepare chiral allylmetals for the reaction with aldehydes. H. C. Brown was the first to report the chiral allylboron reagents for asymmetric allylation reactions with aldehydes. The chiral allylboron reagents were synthesized from the natural product (+)-a-pinene in two steps. The TADDOL ligands developed by Dieter Seebach
Dieter Seebach is a German chemist known for his synthesis of biopolymers and dendrimers, and for his contributions to stereochemistry. He was born on 31 October 1937 in Karlsruhe. He studied chemistry at the University of Karlsruhe (TH) under ...
has been used to prepare chiral allyltitanium compounds for asymmetric allylation with aldehydes. Jim Leighton has developed chiral allysilicon compounds in which the release of ring strain facilitated the stereoselective allylation reaction, 95% to 98% enantiomeric excess could be achieved for a range of achiral aldehydes.Kinnaird, J. W. A.; Ng, P. Y.; Kubota, K.; Wang, X.; Leighton, J. L. J. Am. Chem. Soc. 2002, 124, 7920.

See also
*Macrocyclic stereocontrol
Macrocyclic stereocontrol refers to the directed outcome of a given intermolecular or intramolecular chemical reaction, generally an organic reaction, that is governed by the conformational or geometrical preference of a carbocyclic or heterocyc ...
* Cieplak effect
References
External links
The Evolution of Models for Carbonyl Addition
Evans Group Afternoon Seminar Sarah Siska February 9, 2001 {{DEFAULTSORT:Asymmetric Induction Stereochemistry Induct