|
Macrocyclic Stereocontrol
Macrocyclic stereocontrol refers to the directed outcome of a given intermolecular or intramolecular chemical reaction, generally an organic reaction, that is governed by the conformational or geometrical preference of a carbocyclic or heterocyclic ring, where the ring containing 8 or more atoms. Introduction Stereocontrol for cyclohexane rings is well established in organic chemistry, in large part due to the axial/equatorial preferential positioning of substituents on the ring. Macrocyclic stereocontrol models the substitution and reactions of medium and large rings in organic chemistry, with remote stereogenic elements providing enough conformational influence to direct the outcome of a reaction. Early assumptions towards macrocycles in synthetic chemistry considered them far too floppy to provide any degree of stereochemical or regiochemical control in a reaction. The experiments of W. Clark Still in the late 1970s and 1980s challenged this assumption,Still, W. C.; Galynk ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intermolecular
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction or repulsion which act between atoms and other types of neighbouring particles, e.g. atoms or ions. Intermolecular forces are weak relative to intramolecular forces – the forces which hold a molecule together. For example, the covalent bond, involving sharing electron pairs between atoms, is much stronger than the forces present between neighboring molecules. Both sets of forces are essential parts of force fields frequently used in molecular mechanics. The investigation of intermolecular forces starts from macroscopic observations which indicate the existence and action of forces at a molecular level. These observations include non-ideal-gas thermodynamic behavior reflected by virial coefficients, vapor pressure, viscosity, superficial tension, and absorption data. The first reference to the nature of microscopic forc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activation Energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). Activation energy can be thought of as the magnitude of the potential barrier (sometimes called the energy barrier) separating minima of the potential energy surface pertaining to the initial and final thermodynamic state. For a chemical reaction to proceed at a reasonable rate, the temperature of the system should be high enough such that there exists an appreciable number of molecules with translational energy equal to or greater than the activation energy. The term "activation energy" was introduced in 1889 by the Swedish scientist Svante Arrhenius. Other uses Although less commonly used, activation energy also applies to nuclear reactions and various other physical phenomena. Te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acyclic Stereocontrol with little or no correlation to the business cycle
{{disambig ...
Acyclic may refer to: * In chemistry, a compound which is an open-chain compound, e.g. alkanes and acyclic aliphatic compounds * In mathematics: ** A graph without a cycle, especially *** A directed acyclic graph ** An acyclic complex is a chain complex all of whose homology groups are zero * In economics, an economic indicator An economic indicator is a statistic about an economic activity. Economic indicators allow analysis of economic performance and predictions of future performance. One application of economic indicators is the study of business cycles. Economic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclodecane Configurations
Cyclodecane is a cycloalkane with the chemical formula In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbol ... C10H20. References External links * Cycloalkanes Ten-membered rings {{Hydrocarbon-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
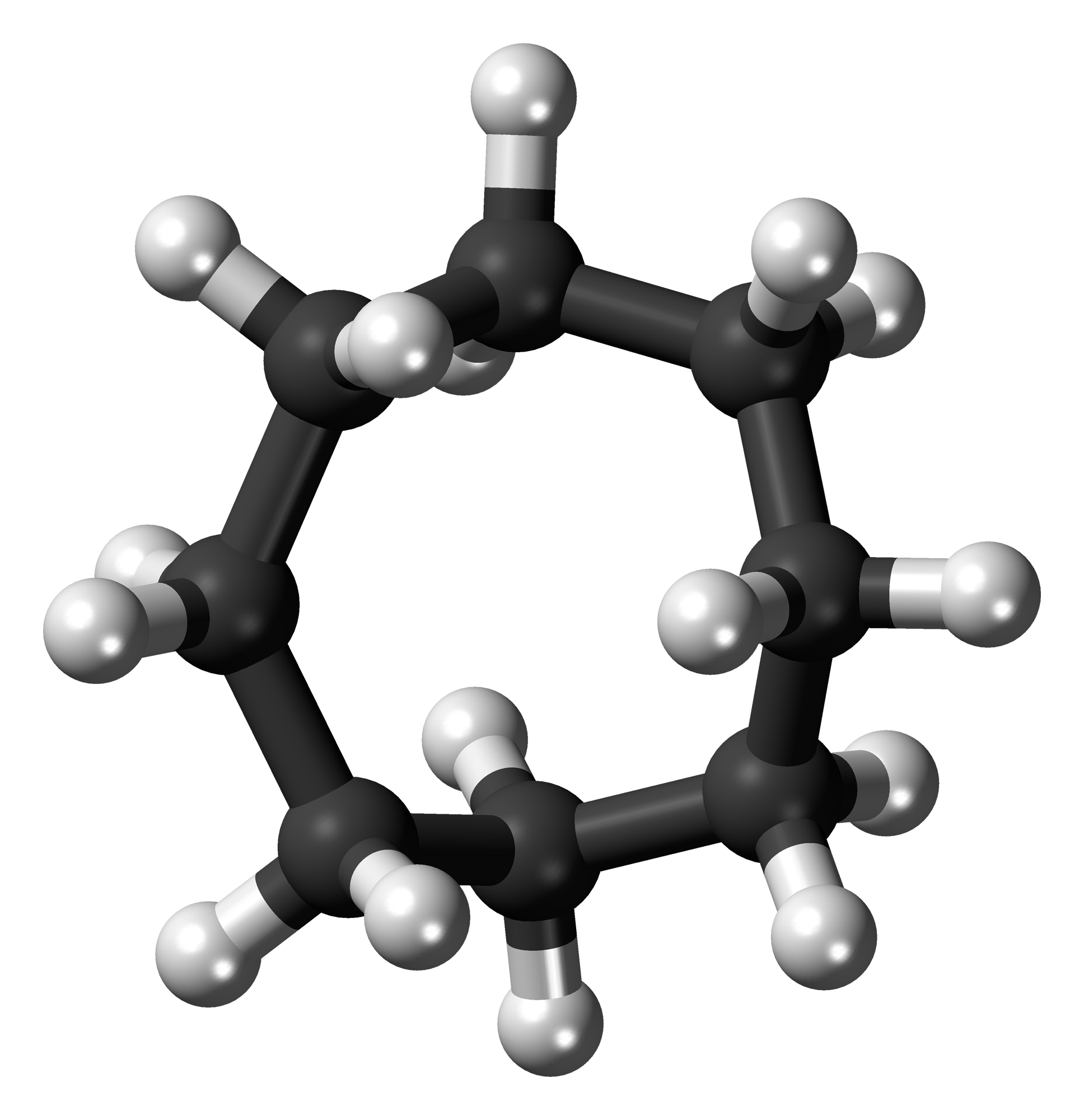
Cyclooctane X-ray Proof
Cyclooctane is a cycloalkane with the molecular formula (CH2)8. It is a simple colourless hydrocarbon, but it is often a reference compound for saturated eight-membered ring compounds in general. Cyclooctane has a camphoraceous odor. Conformations The conformation of cyclooctane has been studied extensively using computational methods. Hendrickson noted that "cyclooctane is unquestionably the conformationally most complex cycloalkane owing to the existence of many conformers of comparable energy". The boat-chair conformation (below) is the most stable form. This conformation was confirmed by Allinger and co-workers. The crown conformation (below) is slightly less stable. Among the many compounds exhibiting the crown conformation (structure II) is S8, elemental sulfur. : Synthesis and reactions The main route to cyclooctane derivatives involves the dimerization of butadiene, catalysed by nickel(0) complexes such as nickel bis(cyclooctadiene). This process affords, among oth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray
An X-ray, or, much less commonly, X-radiation, is a penetrating form of high-energy electromagnetic radiation. Most X-rays have a wavelength ranging from 10 picometers to 10 nanometers, corresponding to frequencies in the range 30 petahertz to 30 exahertz ( to ) and energies in the range 145 eV to 124 keV. X-ray wavelengths are shorter than those of UV rays and typically longer than those of gamma rays. In many languages, X-radiation is referred to as Röntgen radiation, after the German scientist Wilhelm Conrad Röntgen, who discovered it on November 8, 1895. He named it ''X-radiation'' to signify an unknown type of radiation.Novelline, Robert (1997). ''Squire's Fundamentals of Radiology''. Harvard University Press. 5th edition. . Spellings of ''X-ray(s)'' in English include the variants ''x-ray(s)'', ''xray(s)'', and ''X ray(s)''. The most familiar use of X-rays is checking for fractures (broken bones), but X-rays are also used in other ways. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclooctane Sp2 Centers
Cyclooctane is a cycloalkane with the molecular formula (CH2)8. It is a simple colourless hydrocarbon, but it is often a reference compound for saturated eight-membered ring compounds in general. Cyclooctane has a camphoraceous odor. Conformations The conformation of cyclooctane has been studied extensively using computational methods. Hendrickson noted that "cyclooctane is unquestionably the conformationally most complex cycloalkane owing to the existence of many conformers of comparable energy". The boat-chair conformation (below) is the most stable form. This conformation was confirmed by Allinger and co-workers. The crown conformation (below) is slightly less stable. Among the many compounds exhibiting the crown conformation (structure II) is S8, elemental sulfur. : Synthesis and reactions The main route to cyclooctane derivatives involves the dimerization of butadiene, catalysed by nickel(0) complexes such as nickel bis(cyclooctadiene). This process affords, among oth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclooctane Figure 2
Cyclooctane is a cycloalkane with the molecular formula (CH2)8. It is a simple colourless hydrocarbon, but it is often a reference compound for saturated eight-membered ring compounds in general. Cyclooctane has a camphoraceous odor. Conformations The conformation of cyclooctane has been studied extensively using computational methods. Hendrickson noted that "cyclooctane is unquestionably the conformationally most complex cycloalkane owing to the existence of many conformers of comparable energy". The boat-chair conformation (below) is the most stable form. This conformation was confirmed by Allinger and co-workers. The crown conformation (below) is slightly less stable. Among the many compounds exhibiting the crown conformation (structure II) is S8, elemental sulfur. : Synthesis and reactions The main route to cyclooctane derivatives involves the dimerization of butadiene, catalysed by nickel(0) complexes such as nickel bis(cyclooctadiene). This process affords, among oth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |