|
Cieplak Effect
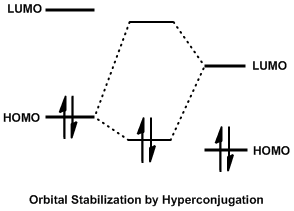
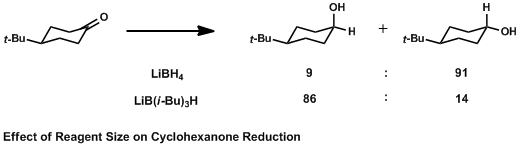
In organic chemistry, the Cieplak effect is a predictive model to rationalize why nucleophiles preferentially add to one face of a carbonyl over another. Proposed by Andrzej Stanislaw Cieplak in 1980, it predicts anomalous results that other models of the time, such as the Cram's rule of asymmetric induction, Cram and Asymmetric induction, Felkin–Anh models, cannot justify. In the Cieplak model, electrons from a neighboring bond delocalize into the forming carbon–nucleophile (C–Nuc) bond, lowering the energy of the transition state and accelerating the rate of reaction.Cieplak, A. S. J. Am. Chem. Soc. 1981, 103, 4540 Whichever bond can best donate its electrons into the C–Nuc bond determines which face of the carbonyl the nucleophile will add to. The nucleophile may be a number of reagents, most commonly organometallic or reducing agents. The Cieplak effect is subtle, and often competes with sterics, solvent effects, counterion complexation of the carbonyl oxygen, and other e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cieplak Effect
In organic chemistry, the Cieplak effect is a predictive model to rationalize why nucleophiles preferentially add to one face of a carbonyl over another. Proposed by Andrzej Stanislaw Cieplak in 1980, it predicts anomalous results that other models of the time, such as the Cram's rule of asymmetric induction, Cram and Asymmetric induction, Felkin–Anh models, cannot justify. In the Cieplak model, electrons from a neighboring bond delocalize into the forming carbon–nucleophile (C–Nuc) bond, lowering the energy of the transition state and accelerating the rate of reaction.Cieplak, A. S. J. Am. Chem. Soc. 1981, 103, 4540 Whichever bond can best donate its electrons into the C–Nuc bond determines which face of the carbonyl the nucleophile will add to. The nucleophile may be a number of reagents, most commonly organometallic or reducing agents. The Cieplak effect is subtle, and often competes with sterics, solvent effects, counterion complexation of the carbonyl oxygen, and other e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |