|
Cieplak Effect
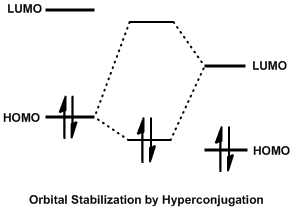
In organic chemistry, the Cieplak effect is a predictive model to rationalize why nucleophiles preferentially add to one face of a carbonyl over another. Proposed by Andrzej Stanislaw Cieplak in 1980, it predicts anomalous results that other models of the time, such as the Cram's rule of asymmetric induction, Cram and Asymmetric induction, Felkin–Anh models, cannot justify. In the Cieplak model, electrons from a neighboring bond delocalize into the forming carbon–nucleophile (C–Nuc) bond, lowering the energy of the transition state and accelerating the rate of reaction.Cieplak, A. S. J. Am. Chem. Soc. 1981, 103, 4540 Whichever bond can best donate its electrons into the C–Nuc bond determines which face of the carbonyl the nucleophile will add to. The nucleophile may be a number of reagents, most commonly organometallic or reducing agents. The Cieplak effect is subtle, and often competes with sterics, solvent effects, counterion complexation of the carbonyl oxygen, and other e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cieplak Effect
In organic chemistry, the Cieplak effect is a predictive model to rationalize why nucleophiles preferentially add to one face of a carbonyl over another. Proposed by Andrzej Stanislaw Cieplak in 1980, it predicts anomalous results that other models of the time, such as the Cram's rule of asymmetric induction, Cram and Asymmetric induction, Felkin–Anh models, cannot justify. In the Cieplak model, electrons from a neighboring bond delocalize into the forming carbon–nucleophile (C–Nuc) bond, lowering the energy of the transition state and accelerating the rate of reaction.Cieplak, A. S. J. Am. Chem. Soc. 1981, 103, 4540 Whichever bond can best donate its electrons into the C–Nuc bond determines which face of the carbonyl the nucleophile will add to. The nucleophile may be a number of reagents, most commonly organometallic or reducing agents. The Cieplak effect is subtle, and often competes with sterics, solvent effects, counterion complexation of the carbonyl oxygen, and other e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical ( in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (included in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anomeric Effect
In organic chemistry, the anomeric effect or Edward-Lemieux effect is a stereoelectronic effect that describes the tendency of heteroatomic substituents adjacent to a heteroatom within a cyclohexane ring to prefer the ''axial'' orientation instead of the less hindered ''equatorial'' orientation that would be expected from steric considerations. This effect was originally observed in pyranose rings by J. T. Edward in 1955 when studying carbohydrate chemistry. The term ''anomeric effect'' was introduced in 1958. The name comes from the term used to designate the lowest-numbered ring carbon of a pyranose, the ''anomeric'' carbon. Isomers that differ only in the configuration at the anomeric carbon are called '' anomers''. The anomers of D-glucopyranose are diastereomers, with the ''beta'' anomer having an OH group pointing up equatorially, and the ''alpha'' anomer having that OH group pointing down axially. The anomeric effect can also be generalized to any cyclohexyl or linear syst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Axial Attack Of Methylenecyclohexane
Axial may refer to: * one of the anatomical directions describing relationships in an animal body * In geometry: :* a geometric term of location :* an axis of rotation Rotation around a fixed axis is a special case of rotational motion. The fixed-axis hypothesis excludes the possibility of an axis changing its orientation and cannot describe such phenomena as wobbling or precession. According to Euler's rota ... * In chemistry, referring to an axial bond * a type of modal frame, in music * axial-flow, a type of fan * the Axial Age in China, India, etc. * Axial Seamount and submarine volcano off Oregon, USA * Axial, Colorado, a ghost town See also * Axiality (other) * Axis (other) {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Axial Reduction Of Substituted Cyclohexanones
Axial may refer to: * one of the anatomical directions describing relationships in an animal body * In geometry: :* a geometric term of location :* an axis of rotation Rotation around a fixed axis is a special case of rotational motion. The fixed-axis hypothesis excludes the possibility of an axis changing its orientation and cannot describe such phenomena as wobbling or precession. According to Euler's rota ... * In chemistry, referring to an axial bond * a type of modal frame, in music * axial-flow, a type of fan * the Axial Age in China, India, etc. * Axial Seamount and submarine volcano off Oregon, USA * Axial, Colorado, a ghost town See also * Axiality (other) * Axis (other) {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methoxy Group
In organic chemistry, a methoxy group is the functional group consisting of a methyl group bound to oxygen. This alkoxy group has the formula . On a benzene ring, the Hammett equation classifies a methoxy substituent at the ''para'' position as an electron-donating group, but as an electron-withdrawing group if at the ''meta'' position. At the ''ortho'' position, steric effects are likely to cause a significant alteration in the Hammett equation prediction which otherwise follows the same trend as that of the ''para'' position. Occurrence The simplest of methoxy compounds are methanol and dimethyl ether. Other methoxy ethers include anisole and vanillin. Many alkoxides contain methoxy groups, e.g. tetramethyl orthosilicate and titanium methoxide. Such compounds are often classified as methoxides. Esters with a methoxy group can be referred to as methyl esters, and the —COOCH3 substituent is called a methoxycarbonyl. Biosynthesis In nature, methoxy groups are found on nucleosi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Axial Vs Equatorial Approach In The Cieplak Model
Axial may refer to: * one of the anatomical directions describing relationships in an animal body * In geometry: :* a geometric term of location :* an axis of rotation Rotation around a fixed axis is a special case of rotational motion. The fixed-axis hypothesis excludes the possibility of an axis changing its orientation and cannot describe such phenomena as wobbling or precession. According to Euler's rota ... * In chemistry, referring to an axial bond * a type of modal frame, in music * axial-flow, a type of fan * the Axial Age in China, India, etc. * Axial Seamount and submarine volcano off Oregon, USA * Axial, Colorado, a ghost town See also * Axiality (other) * Axis (other) {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Donors
In chemistry, an electron donor is a chemical entity that donates electrons to another compound. It is a reducing agent that, by virtue of its donating electrons, is itself oxidized in the process. Typical reducing agents undergo permanent chemical alteration through covalent or ionic reaction chemistry. This results in the complete and irreversible transfer of one or more electrons. In many chemical circumstances, however, the transfer of electronic charge to an electron acceptor may be only fractional, meaning an electron is not completely transferred, but results in an electron resonance between the donor and acceptor. This leads to the formation of charge transfer complexes in which the components largely retain their chemical identities. The electron donating power of a donor molecule is measured by its ionization potential which is the energy required to remove an electron from the highest occupied molecular orbital (HOMO). The overall energy balance (ΔE), i.e., energy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non- stereospecific creation of a new stereocenter or during a non-stereospecific transformation of a pre-existing one. The selectivity arises from differences in steric and electronic effects in the mechanistic pathways leading to the different products. Stereoselectivity can vary in degree but it can never be total since the activation energy difference between the two pathways is finite. Both products are at least possible and merely differ in amount. However, in favorable cases, the minor stereoisomer may not be detectable by the analytic methods used. An enantioselective reaction is one in which one enantiomer is formed in preference to the other, in a reaction that creates an optically active product from an achiral starting material, using either a chiral catalyst, an enzyme or a chiral reagent. The degree of selectivity is meas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
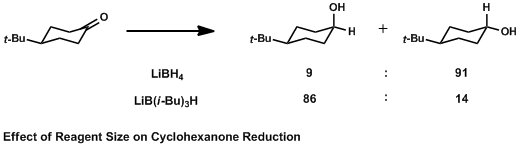
Effect Of Reagent Size On Cyclohexanone Reduction
Effect may refer to: * A result or change of something ** List of effects ** Cause and effect, an idiom describing causality Pharmacy and pharmacology * Drug effect, a change resulting from the administration of a drug ** Therapeutic effect, a beneficial change in medical condition, often caused by a drug ** Adverse effect or side effect, an unwanted change in medical condition caused by a drug In media * Special effect, an artificial illusion ** Sound effect, an artificially created or enhanced sound ** Visual effects, artificially created or enhanced images * Audio signal processing ** Effects unit, a device used to manipulate electronic sound *** Effects pedal, a small device attached to an instrument to modify its sound Other uses * Effects, one's personal property or belongings * Effects (G.I. Joe), a fictional character in the G.I. Joe universe * ''Effects'' (film), a 2005 film * Effect size, a measure of the strength of a relationship between two variables * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. For inorganic chemists, hydrides refer to compounds and ions in which hydrogen is covalently attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed. Almost all of the elements form binary compounds with hydrogen, the exceptions being He, Ne, Ar, Kr, Pm, Os, Ir, Rn, Fr, and Ra. Exotic molecules such as positronium hydride have also been made. Bonds Bonds between hydrogen and the other elements range from highly to somewhat covalent. Some hydrides, e.g. boron hydrides, do not conform to classical electron-counting rules and the bonding is described in terms of multi-centered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |