Curium on:
[Wikipedia]
[Google]
[Amazon]
Curium is a

 Though curium had likely been produced in previous nuclear experiments as well as the
Though curium had likely been produced in previous nuclear experiments as well as the ^_Pu + ^_He -> ^_Cm + ^_n
Curium-242 was unambiguously identified by the characteristic energy of the α-particles emitted during the decay:
: ^_Cm -> ^_Pu + ^_He
The ^_Pu + ^_He -> ^_Cm + 3^_n
The α-decay half-life of 240Cm was correctly determined as 26.7 days.
The discovery of curium and americium in 1944 was closely related to the
 A synthetic, radioactive element, curium is a hard, dense metal with a silvery-white appearance and physical and chemical properties resembling
A synthetic, radioactive element, curium is a hard, dense metal with a silvery-white appearance and physical and chemical properties resembling
 Curium ion in solution almost always has a +3
Curium ion in solution almost always has a +3
All isotopes 242Cm-248Cm, and 250Cm, undergo a self-sustaining
"Evaluation of nuclear criticality safety. data and limits for actinides in transport"
, p. 16 The mixed-oxide (MOX) fuel, which is to be used in power reactors, should contain little or no curium because The adjacent table lists the
The adjacent table lists the
 The longest-lived isotope, 247Cm, has half-life 15.6 million years; so any primordial curium, that is, present on Earth when it formed, should have decayed by now. Its past presence as an
The longest-lived isotope, 247Cm, has half-life 15.6 million years; so any primordial curium, that is, present on Earth when it formed, should have decayed by now. Its past presence as an
(in German) Analysis of the debris at the test site of the
 Most synthesis routines yield a mix of actinide isotopes as
Most synthesis routines yield a mix of actinide isotopes as
4CmO2 -> .
Or, Cm2O3 can be obtained by reducing CmO2 with molecular 2CmO2 + H2 -> Cm2O3 + H2O
Also, a number of ternary oxides of the type M(II)CmO3 are known, where M stands for a divalent metal, such as barium.
Thermal oxidation of trace quantities of curium hydride (CmH2–3) has been reported to give a volatile form of CmO2 and the volatile trioxide CmO3, one of two known examples of the very rare +6 state for curium. Another observed species was reported to behave similar to a supposed plutonium tetroxide and was tentatively characterized as CmO4, with curium in the extremely rare +8 state; but new experiments seem to indicate that CmO4 does not exist, and have cast doubt on the existence of PuO4 as well.
Curium
, Chapter Nine in ''Radioanalytical Chemistry'', Springer, 2004, pp. 1420–1421. ,
 Organometallic complexes analogous to
Organometallic complexes analogous to
, G.L. Kulcinski, NEEP 602 Course Notes (Spring 2000), Nuclear Power in Space, University of Wisconsin Fusion Technology Institute (see last page) A more promising use of 242Cm is for making 238Pu, a better radioisotope for thermoelectric generators such as in heart pacemakers. The alternate routes to 238Pu use the (n,γ) reaction of 237Np, or
, Los Alamos National Laboratory An elaborate APXS setup has a sensor head containing six curium sources with a total decay rate of several tens of millicuries (roughly one
Application of Partitioning/Transmutation of Radioactive Materials in Radioactive Waste Management
, Nuclear Research Centre of Belgium Sck/Cen, Mol, Belgium, September 2001. Such a procedure involves several steps, where curium is first separated and then converted by neutron bombardment in special reactors to short-lived nuclides. This procedure,
The radiochemistry of americium and curium
University of California, Los Alamos, California, 1960
at ''
NLM Hazardous Substances Databank – Curium, Radioactive
{{Marie & Pierre Curie Chemical elements Chemical elements with double hexagonal close-packed structure Actinides American inventions Synthetic elements Marie Curie Pierre Curie
transuranic
The transuranium elements (also known as transuranic elements) are the chemical elements with atomic numbers greater than 92, which is the atomic number of uranium. All of these elements are unstable and decay radioactively into other elements. ...
, radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consi ...
chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
with the symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
Cm and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
96. This actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The inform ...
element was named after eminent scientists Marie
Marie may refer to:
People Name
* Marie (given name)
* Marie (Japanese given name)
* Marie (murder victim), girl who was killed in Florida after being pushed in front of a moving vehicle in 1973
* Marie (died 1759), an enslaved Cree person in Tr ...
and Pierre Curie
Pierre Curie ( , ; 15 May 1859 – 19 April 1906) was a French physicist, a pioneer in crystallography, magnetism, piezoelectricity, and radioactivity. In 1903, he received the Nobel Prize in Physics with his wife, Marie Curie, and Henri Becqu ...
, both known for their research on radioactivity. Curium was first intentionally made by the team of Glenn T. Seaborg
Glenn Theodore Seaborg (; April 19, 1912February 25, 1999) was an American chemist whose involvement in the synthesis, discovery and investigation of ten transuranium elements earned him a share of the 1951 Nobel Prize in Chemistry. His work i ...
, Ralph A. James, and Albert Ghiorso
Albert Ghiorso (July 15, 1915 – December 26, 2010) was an American nuclear scientist and co-discoverer of a record 12 chemical elements on the periodic table. His research career spanned six decades, from the early 1940s to the late 1990s.
Biog ...
in 1944, using the cyclotron
A cyclotron is a type of particle accelerator invented by Ernest O. Lawrence in 1929–1930 at the University of California, Berkeley, and patented in 1932. Lawrence, Ernest O. ''Method and apparatus for the acceleration of ions'', filed: Janu ...
at Berkeley
Berkeley most often refers to:
*Berkeley, California, a city in the United States
**University of California, Berkeley, a public university in Berkeley, California
* George Berkeley (1685–1753), Anglo-Irish philosopher
Berkeley may also refer ...
. They bombarded the newly discovered element plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibi ...
(the isotope 239Pu) with alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be pr ...
s. This was then sent to the Metallurgical Laboratory at University of Chicago
The University of Chicago (UChicago, Chicago, U of C, or UChi) is a private research university in Chicago, Illinois. Its main campus is located in Chicago's Hyde Park neighborhood. The University of Chicago is consistently ranked among the b ...
where a tiny sample of curium was eventually separated and identified. The discovery was kept secret until after the end of World War II
World War II or the Second World War, often abbreviated as WWII or WW2, was a world war that lasted from 1939 to 1945. It involved the vast majority of the world's countries—including all of the great powers—forming two opposin ...
. The news was released to the public in November 1947. Most curium is produced by bombarding uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
or plutonium with neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons beh ...
s in nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat fr ...
s – one tonne
The tonne ( or ; symbol: t) is a unit of mass equal to 1000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton to distinguish it from the non-metric units of the short ton ( United State ...
of spent nuclear fuel contains ~20 grams of curium.
Curium is a hard, dense, silvery metal with a high melting and boiling point for an actinide. It is paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
at ambient conditions, but becomes antiferromagnetic
In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usually related to the spins of electrons, align in a regular pattern with neighboring spins (on different sublattices) pointing in opposite directions. ...
upon cooling, and other magnetic transitions are also seen in many curium compounds. In compounds, curium usually has valence +3 and sometimes +4; the +3 valence is predominant in solutions. Curium readily oxidizes, and its oxides are a dominant form of this element. It forms strongly fluorescent
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, tha ...
complexes with various organic compounds, but there is no evidence of its incorporation into bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among ...
and archaea
Archaea ( ; singular archaeon ) is a domain of single-celled organisms. These microorganisms lack cell nuclei and are therefore prokaryotes. Archaea were initially classified as bacteria, receiving the name archaebacteria (in the Archaebac ...
. If it gets into the human body, curium accumulates in bones, lungs, and liver, where it promotes cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
.
All known isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) ...
s of curium are radioactive and have small critical mass
In nuclear engineering, a critical mass is the smallest amount of fissile material needed for a sustained nuclear chain reaction. The critical mass of a fissionable material depends upon its nuclear properties (specifically, its nuclear fi ...
for a nuclear chain reaction
In nuclear physics, a nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions, thus leading to the possibility of a self-propagating series of these reactions. The specific nu ...
. They mostly emit α-particles; radioisotope thermoelectric generator
A radioisotope thermoelectric generator (RTG, RITEG), sometimes referred to as a radioisotope power system (RPS), is a type of nuclear battery that uses an array of thermocouples to convert the heat released by the decay of a suitable radioacti ...
s can use the heat from this process, but this is hindered by the rarity and high cost of curium. Curium is used in making heavier actinides and the 238Pu radionuclide
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transfer ...
for power sources in artificial cardiac pacemaker
An artificial cardiac pacemaker (or artificial pacemaker, so as not to be confused with the natural cardiac pacemaker) or pacemaker is a medical device that generates electrical impulses delivered by electrodes to the chambers of the heart ei ...
s and RTGs for spacecraft. It served as the α-source in the alpha particle X-ray spectrometer
:''APXS is also an abbreviation for APache eXtenSion tool, an extension for Apache web servers.''
An alpha particle X-ray spectrometer (APXS) is a spectrometer that analyses the chemical element composition of a sample from scattered alpha part ...
s of several space probes, including the ''Sojourner
A sojourner is a person who resides temporarily in a place.
Sojourner may also refer to:
* Sojourner Truth (1797–1883), abolitionist and women's rights activist
* Albert Sojourner (1872–1951), member of the Mississippi House of Representative ...
'', ''Spirit
Spirit or spirits may refer to:
Liquor and other volatile liquids
* Spirits, a.k.a. liquor, distilled alcoholic drinks
* Spirit or tincture, an extract of plant or animal material dissolved in ethanol
* Volatile (especially flammable) liquids, ...
'', ''Opportunity
Opportunity may refer to:
Places
* Opportunity, Montana, an unincorporated community, United States
* Opportunity, Nebraska, an unincorporated community, United States
* Opportunity, Washington, a former census-designated place, United States
* ...
'', and ''Curiosity
Curiosity (from Latin '' cūriōsitās'', from ''cūriōsus'' "careful, diligent, curious", akin to ''cura'' "care") is a quality related to inquisitive thinking such as exploration, investigation, and learning, evident by observation in humans ...
'' Mars rover
A Mars rover is a motor vehicle designed to travel on the surface of Mars. Rovers have several advantages over stationary landers: they examine more territory, they can be directed to interesting features, they can place themselves in sunny pos ...
s and the Philae lander on comet
A comet is an icy, small Solar System body that, when passing close to the Sun, warms and begins to release gases, a process that is called outgassing. This produces a visible atmosphere or coma, and sometimes also a tail. These phenomena ar ...
67P/Churyumov–Gerasimenko
67P/Churyumov–Gerasimenko (abbreviated as 67P or 67P/C–G) is a Jupiter-family comet, originally from the Kuiper belt, with a current orbital period of 6.45 years, a rotation period of approximately 12.4 hours and a maximum velocity of . Chu ...
, to analyze the composition and structure of the surface.
History

 Though curium had likely been produced in previous nuclear experiments as well as the
Though curium had likely been produced in previous nuclear experiments as well as the natural nuclear fission reactor
A natural nuclear fission reactor is a uranium deposit where self-sustaining nuclear chain reactions occur. The conditions under which a natural nuclear reactor could exist had been predicted in 1956 by Japanese American chemist Paul Kuroda. ...
at Oklo, Gabon, it was first intentionally synthesized, isolated and identified in 1944, at University of California, Berkeley
The University of California, Berkeley (UC Berkeley, Berkeley, Cal, or California) is a public land-grant research university in Berkeley, California. Established in 1868 as the University of California, it is the state's first land-grant u ...
, by Glenn T. Seaborg
Glenn Theodore Seaborg (; April 19, 1912February 25, 1999) was an American chemist whose involvement in the synthesis, discovery and investigation of ten transuranium elements earned him a share of the 1951 Nobel Prize in Chemistry. His work i ...
, Ralph A. James, and Albert Ghiorso
Albert Ghiorso (July 15, 1915 – December 26, 2010) was an American nuclear scientist and co-discoverer of a record 12 chemical elements on the periodic table. His research career spanned six decades, from the early 1940s to the late 1990s.
Biog ...
. In their experiments, they used a cyclotron
A cyclotron is a type of particle accelerator invented by Ernest O. Lawrence in 1929–1930 at the University of California, Berkeley, and patented in 1932. Lawrence, Ernest O. ''Method and apparatus for the acceleration of ions'', filed: Janu ...
.
Curium was chemically identified at the Metallurgical Laboratory (now Argonne National Laboratory), University of Chicago
The University of Chicago (UChicago, Chicago, U of C, or UChi) is a private research university in Chicago, Illinois. Its main campus is located in Chicago's Hyde Park neighborhood. The University of Chicago is consistently ranked among the b ...
. It was the third transuranium element
The transuranium elements (also known as transuranic elements) are the chemical elements with atomic numbers greater than 92, which is the atomic number of uranium. All of these elements are unstable and decay radioactively into other elements. ...
to be discovered even though it is the fourth in the series – the lighter element americium
Americium is a synthetic radioactive chemical element with the symbol Am and atomic number 95. It is a transuranic member of the actinide series, in the periodic table located under the lanthanide element europium, and thus by analogy was na ...
was still unknown.
The sample was prepared as follows: first plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibi ...
nitrate solution was coated on a platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Platinu ...
foil of ~0.5 cm2 area, the solution was evaporated and the residue was converted into plutonium(IV) oxide
Plutonium(IV) oxide or (plutonia) is the chemical compound with the formula Pu O2. This high melting-point solid is a principal compound of plutonium. It can vary in color from yellow to olive green, depending on the particle size, temperature a ...
(PuO2) by annealing. Following cyclotron irradiation of the oxide, the coating was dissolved with nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitri ...
and then precipitated as the hydroxide using concentrated aqueous ammonia solution
Ammonia solution, also known as ammonia water, ammonium hydroxide, ammoniacal liquor, ammonia liquor, aqua ammonia, aqueous ammonia, or (inaccurately) ammonia, is a solution of ammonia in water. It can be denoted by the symbols NH3(aq). Although ...
. The residue was dissolved in perchloric acid
Perchloric acid is a mineral acid with the formula H Cl O4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid, nitric acid and hydrochloric acid. It is a powerful oxidizer when hot, but aqueous s ...
, and further separation was done by ion exchange
Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, ...
to yield a certain isotope of curium. The separation of curium and americium was so painstaking that the Berkeley group initially called those elements '' pandemonium'' (from Greek for ''all demons'' or ''hell'') and '' delirium'' (from Latin for ''madness'').
Curium-242 was made in July–August 1944 by bombarding 239Pu with α-particles to produce curium with the release of a neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons beh ...
:
: half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ato ...
of this alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an at ...
was first measured as 150 days and then corrected to 162.8 days.
Another isotope 240Cm was produced in a similar reaction in March 1945:
: Manhattan Project
The Manhattan Project was a research and development undertaking during World War II that produced the first nuclear weapons. It was led by the United States with the support of the United Kingdom and Canada. From 1942 to 1946, the project w ...
, so the results were confidential and declassified only in 1945. Seaborg leaked the synthesis of the elements 95 and 96 on the U.S. radio show for children, the Quiz Kids
''Quiz Kids'' is a radio and TV series originally broadcast in the 1940s and 1950s. Created by Chicago public relations and advertising man Louis G. Cowan, and originally sponsored by Alka-Seltzer, the series was first broadcast on NBC from Chic ...
, five days before the official presentation at an American Chemical Society
The American Chemical Society (ACS) is a scientific society based in the United States that supports scientific inquiry in the field of chemistry. Founded in 1876 at New York University, the ACS currently has more than 155,000 members at all d ...
meeting on November 11, 1945, when one listener asked if any new transuranic element beside plutonium and neptunium
Neptunium is a chemical element with the symbol Np and atomic number 93. A radioactive actinide metal, neptunium is the first transuranic element. Its position in the periodic table just after uranium, named after the planet Uranus, led to it bein ...
had been discovered during the war. The discovery of curium (242Cm and 240Cm), its production, and its compounds was later patented listing only Seaborg as the inventor.
The element was named after Marie Curie
Marie Salomea Skłodowska–Curie ( , , ; born Maria Salomea Skłodowska, ; 7 November 1867 – 4 July 1934) was a Polish and naturalized-French physicist and chemist who conducted pioneering research on radioactivity. She was the first ...
and her husband Pierre Curie
Pierre Curie ( , ; 15 May 1859 – 19 April 1906) was a French physicist, a pioneer in crystallography, magnetism, piezoelectricity, and radioactivity. In 1903, he received the Nobel Prize in Physics with his wife, Marie Curie, and Henri Becqu ...
, who are known for discovering radium
Radium is a chemical element with the symbol Ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rather t ...
and for their work in radioactivity. It followed the example of gadolinium
Gadolinium is a chemical element with the symbol Gd and atomic number 64. Gadolinium is a silvery-white metal when oxidation is removed. It is only slightly malleable and is a ductile rare-earth element. Gadolinium reacts with atmospheric oxygen ...
, a lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttr ...
element above curium in the periodic table, which was named after the explorer of rare-earth element
The rare-earth elements (REE), also called the rare-earth metals or (in context) rare-earth oxides or sometimes the lanthanides ( yttrium and scandium are usually included as rare earths), are a set of 17 nearly-indistinguishable lustrous silv ...
s Johan Gadolin
Johan Gadolin (5 June 176015 August 1852) was a Finnish chemist, physicist and mineralogist. Gadolin discovered a " new earth" containing the first rare-earth compound yttrium, which was later determined to be a chemical element. He is also ...
:
::''"As the name for the element of atomic number 96 we should like to propose "curium", with symbol Cm. The evidence indicates that element 96 contains seven 5f electrons and is thus analogous to the element gadolinium, with its seven 4f electrons in the regular rare earth series. On this basis element 96 is named after the Curies in a manner analogous to the naming of gadolinium, in which the chemist Gadolin was honored."''
The first curium samples were barely visible, and were identified by their radioactivity. Louis Werner and Isadore Perlman made the first substantial sample of 30 µg curium-242 hydroxide at University of California, Berkeley in 1947 by bombarding americium
Americium is a synthetic radioactive chemical element with the symbol Am and atomic number 95. It is a transuranic member of the actinide series, in the periodic table located under the lanthanide element europium, and thus by analogy was na ...
-241 with neutrons.Hammond C. R. "The elements" in Macroscopic amounts of curium(III) fluoride were obtained in 1950 by W. W. T. Crane, J. C. Wallmann and B. B. Cunningham. Its magnetic susceptibility was very close to that of GdF3 providing the first experimental evidence for the +3 valence of curium in its compounds. Curium metal was produced only in 1951 by reduction of CmF3 with barium.
Characteristics
Physical

 A synthetic, radioactive element, curium is a hard, dense metal with a silvery-white appearance and physical and chemical properties resembling
A synthetic, radioactive element, curium is a hard, dense metal with a silvery-white appearance and physical and chemical properties resembling gadolinium
Gadolinium is a chemical element with the symbol Gd and atomic number 64. Gadolinium is a silvery-white metal when oxidation is removed. It is only slightly malleable and is a ductile rare-earth element. Gadolinium reacts with atmospheric oxygen ...
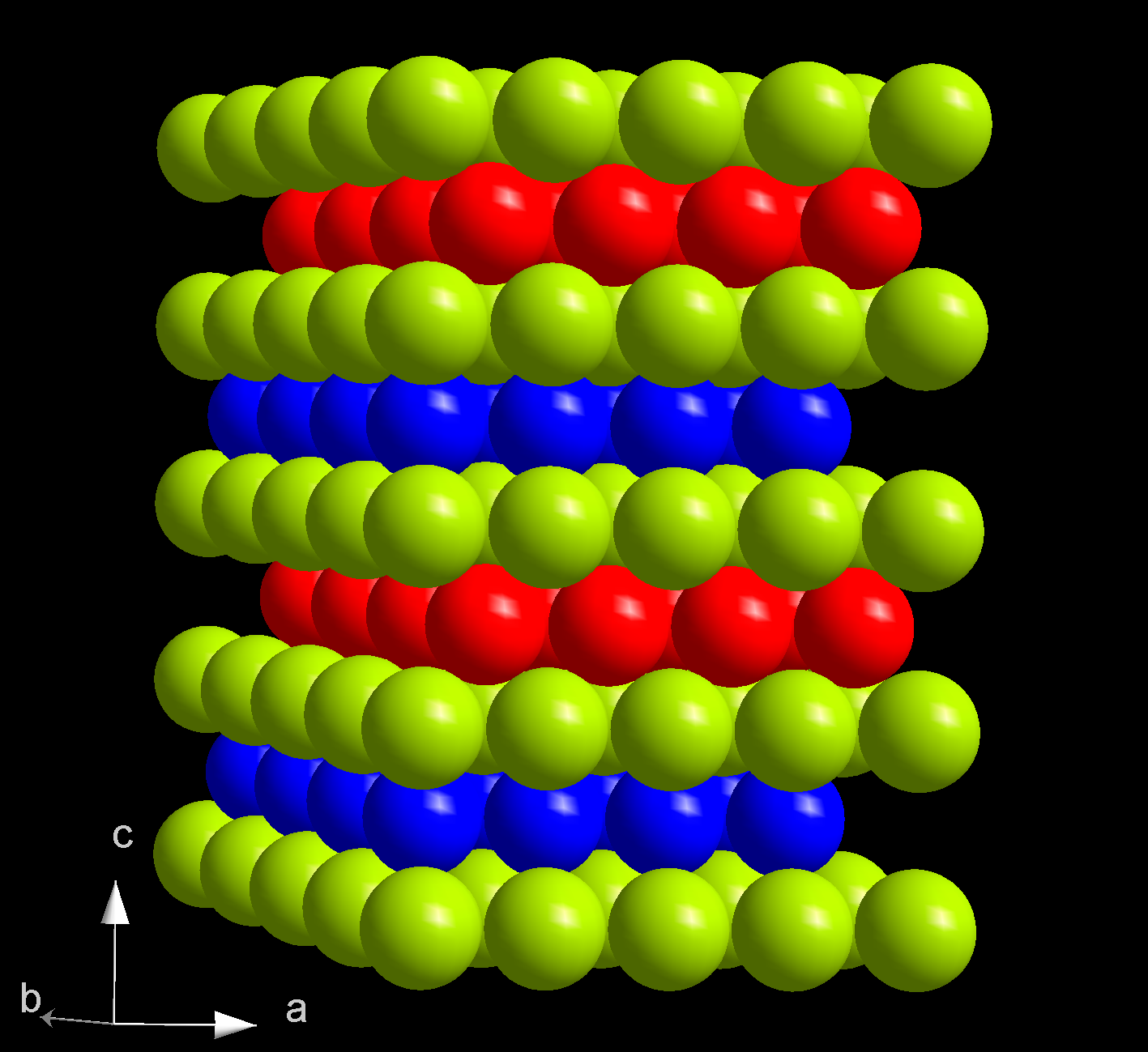
. Its melting point of 1344 °C is significantly higher than that of the previous elements neptunium (637 °C), plutonium (639 °C) and americium (1176 °C). In comparison, gadolinium melts at 1312 °C. Curium boils at 3556 °C. With a density of 13.52 g/cm3, curium is lighter than neptunium (20.45 g/cm3) and plutonium (19.8 g/cm3), but heavier than most other metals. Of two crystalline forms of curium, α-Cm is more stable at ambient conditions. It has a hexagonal symmetry, space group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it unchan ...
P63/mmc, lattice parameters ''a'' = 365 pm and ''c'' = 1182 pm, and four formula unit
In chemistry, a formula unit is the empirical formula of any ionic or covalent network solid compound used as an independent entity for stoichiometric calculations. It is the lowest whole number ratio of ions represented in an ionic compound. E ...
s per unit cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector, for example) does not necessaril ...
. The crystal consists of double-hexagonal close packing
In geometry, close-packing of equal spheres is a dense arrangement of congruent spheres in an infinite, regular arrangement (or lattice). Carl Friedrich Gauss proved that the highest average density – that is, the greatest fraction of space occu ...
with the layer sequence ABAC and so is isotypic with α-lanthanum. At pressure >23 GPa
Grading in education is the process of applying standardized measurements for varying levels of achievements in a course. Grades can be assigned as letters (usually A through F), as a range (for example, 1 to 6), as a percentage, or as a numbe ...
, at room temperature, α-Cm becomes β-Cm, which has face-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties of ...
symmetry, space group Fmm and lattice constant ''a'' = 493 pm. On further compression to 43 GPa, curium becomes an orthorhombic γ-Cm structure similar to α-uranium, with no further transitions observed up to 52 GPa. These three curium phases are also called Cm I, II and III.
Curium has peculiar magnetic properties. Its neighbor element americium shows no deviation from Curie-Weiss paramagnetism
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
in the entire temperature range, but α-Cm transforms to an antiferromagnetic
In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usually related to the spins of electrons, align in a regular pattern with neighboring spins (on different sublattices) pointing in opposite directions. ...
state upon cooling to 65–52 K, and β-Cm exhibits a ferrimagnetic
A ferrimagnetic material is a material that has populations of atoms with opposing magnetic moments, as in antiferromagnetism, but these moments are unequal in magnitude so a spontaneous magnetization remains. This can for example occur when ...
transition at ~205 K. Curium pnictides show ferromagnetic
Ferromagnetism is a property of certain materials (such as iron) which results in a large observed magnetic permeability, and in many cases a large magnetic coercivity allowing the material to form a permanent magnet. Ferromagnetic materials ...
transitions upon cooling: 244CmN and 244CmAs at 109 K, 248CmP at 73 K and 248CmSb at 162 K. The lanthanide analog of curium, gadolinium, and its pnictides, also show magnetic transitions upon cooling, but the transition character is somewhat different: Gd and GdN become ferromagnetic, and GdP, GdAs and GdSb show antiferromagnetic ordering.
In accordance with magnetic data, electrical resistivity of curium increases with temperature – about twice between 4 and 60 K – and then is nearly constant up to room temperature. There is a significant increase in resistivity over time (~) due to self-damage of the crystal lattice by alpha decay. This makes uncertain the true resistivity of curium (~). Curium's resistivity is similar to that of gadolinium, and the actinides plutonium and neptunium, but significantly higher than that of americium, uranium, polonium
Polonium is a chemical element with the symbol Po and atomic number 84. Polonium is a chalcogen. A rare and highly radioactive metal with no stable isotopes, polonium is chemically similar to selenium and tellurium, though its metallic character ...
and thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high me ...
.
Under ultraviolet illumination, curium(III) ions show strong and stable yellow-orange fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, tha ...
with a maximum in the range of 590–640 nm depending on their environment. The fluorescence originates from the transitions from the first excited state 6D7/2 and the ground state 8S7/2. Analysis of this fluorescence allows monitoring interactions between Cm(III) ions in organic and inorganic complexes.Bünzli, J.-C. G. and Choppin, G. R. ''Lanthanide probes in life, chemical, and earth sciences: theory and practice'', Elsevier, Amsterdam, 1989
Chemical
 Curium ion in solution almost always has a +3
Curium ion in solution almost always has a +3 oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
, the most stable oxidation state for curium. A +4 oxidation state is seen mainly in a few solid phases, such as CmO2 and CmF4. Aqueous curium(IV) is only known in the presence of strong oxidizers such as potassium persulfate
Potassium persulfate is the inorganic compound with the formula K2 S2O8. Also known as potassium peroxydisulfate, it is a white solid that is sparingly soluble in cold water, but dissolves better in warm water. This salt is a powerful oxidant, c ...
, and is easily reduced to curium(III) by radiolysis and even by water itself. Chemical behavior of curium is different from the actinides thorium and uranium, and is similar to americium and many lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttr ...
s. In aqueous solution, the Cm3+ ion is colorless to pale green;Greenwood, p. 1265 Cm4+ ion is pale yellow.Holleman, p. 1956 The optical absorption of Cm3+ ion contains three sharp peaks at 375.4, 381.2 and 396.5 nm and their strength can be directly converted into the concentration of the ions. The +6 oxidation state has only been reported once in solution in 1978, as the curyl ion (): this was prepared from beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
of americium-242 in the americium(V) ion . Failure to get Cm(VI) from oxidation of Cm(III) and Cm(IV) may be due to the high Cm4+/Cm3+ ionization potential
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule ...
and the instability of Cm(V).
Curium ions are hard Lewis acids and thus form most stable complexes with hard bases. The bonding is mostly ionic, with a small covalent component. Curium in its complexes commonly exhibits a 9-fold coordination environment, within a tricapped trigonal prismatic geometry.
Isotopes
About 19radioisotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferr ...
s and 7 nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy excited state, higher energy levels than in the ground state of the same nucleus. "Metastable" describes nuclei whose excited ...
s, 233Cm to 251Cm, are known; none are stable
A stable is a building in which livestock, especially horses, are kept. It most commonly means a building that is divided into separate stalls for individual animals and livestock. There are many different types of stables in use today; the ...
. The longest half-lives are 15.6 million years (247Cm) and 348,000 years (248Cm). Other long-lived ones are 245Cm (8500 years), 250Cm (8300 years) and 246Cm (4760 years). Curium-250 is unusual: it mostly (~86%) decays by spontaneous fission
Spontaneous fission (SF) is a form of radioactive decay that is found only in very heavy chemical elements. The nuclear binding energy of the elements reaches its maximum at an atomic mass number of about 56 (e.g., iron-56); spontaneous breakdo ...
. The most commonly used isotopes are 242Cm and 244Cm with the half-lives 162.8 days and 18.1 years, respectively.
nuclear chain reaction
In nuclear physics, a nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions, thus leading to the possibility of a self-propagating series of these reactions. The specific nu ...
and thus in principle can be a nuclear fuel in a reactor. As in most transuranic elements, nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radio ...
cross section is especially high for the odd-mass curium isotopes 243Cm, 245Cm and 247Cm. These can be used in thermal-neutron reactor
A thermal-neutron reactor is a nuclear reactor that uses slow or thermal neutrons. ("Thermal" does not mean hot in an absolute sense, but means in thermal equilibrium with the medium it is interacting with, the reactor's fuel, moderator and struct ...
s, whereas a mixture of curium isotopes is only suitable for fast breeder reactors since the even-mass isotopes are not fissile in a thermal reactor and accumulate as burn-up increases.Institut de Radioprotection et de Sûreté Nucléaire"Evaluation of nuclear criticality safety. data and limits for actinides in transport"
, p. 16 The mixed-oxide (MOX) fuel, which is to be used in power reactors, should contain little or no curium because
neutron activation
Neutron activation is the process in which neutron radiation induces radioactivity in materials, and occurs when atomic nuclei capture free neutrons, becoming heavier and entering excited states. The excited nucleus decays immediately by emit ...
of 248Cm will create californium
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding ...
. Californium is a strong neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons beh ...
emitter, and would pollute the back end of the fuel cycle and increase the dose to reactor personnel. Hence, if minor actinide
The minor actinides are the actinide elements in used nuclear fuel other than uranium and plutonium, which are termed the major actinides. The minor actinides include neptunium (element 93), americium (element 95), curium (element 96), berkeliu ...
s are to be used as fuel in a thermal neutron reactor, the curium should be excluded from the fuel or placed in special fuel rods where it is the only actinide present.
critical mass
In nuclear engineering, a critical mass is the smallest amount of fissile material needed for a sustained nuclear chain reaction. The critical mass of a fissionable material depends upon its nuclear properties (specifically, its nuclear fi ...
es for curium isotopes for a sphere, without moderator or reflector. With a metal reflector (30 cm of steel), the critical masses of the odd isotopes are about 3–4 kg. When using water (thickness ~20–30 cm) as the reflector, the critical mass can be as small as 59 gram for 245Cm, 155 gram for 243Cm and 1550 gram for 247Cm. There is significant uncertainty in these critical mass values. While it is usually on the order of 20%, the values for 242Cm and 246Cm were listed as large as 371 kg and 70.1 kg, respectively, by some research groups.
Curium is not currently used as nuclear fuel due to its low availability and high price. 245Cm and 247Cm have very small critical mass and so could be used in tactical nuclear weapon
A tactical nuclear weapon (TNW) or non-strategic nuclear weapon (NSNW) is a nuclear weapon that is designed to be used on a battlefield in military situations, mostly with friendly forces in proximity and perhaps even on contested friendly territo ...
s, but none are known to have been made. Curium-243 is not suitable for such, due to its short half-life and strong α emission, which would cause excessive heat. Curium-247 would be highly suitable due to its long half-life, which is 647 times longer than plutonium-239
Plutonium-239 (239Pu or Pu-239) is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 is also used for that purpose. Plutonium-239 is also one of the three main ...
(used in many existing nuclear weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission (fission bomb) or a combination of fission and fusion reactions ( thermonuclear bomb), producing a nuclear explosion. Both bom ...
s).
Occurrence
 The longest-lived isotope, 247Cm, has half-life 15.6 million years; so any primordial curium, that is, present on Earth when it formed, should have decayed by now. Its past presence as an
The longest-lived isotope, 247Cm, has half-life 15.6 million years; so any primordial curium, that is, present on Earth when it formed, should have decayed by now. Its past presence as an extinct radionuclide
An extinct radionuclide is a radionuclide that was formed by nucleosynthesis before the formation of the Solar System, about 4.6 billion years ago, but has since decayed to virtually zero abundance and is no longer detectable as a primordial nuc ...
is detectable as an excess of its primordial, long-lived daughter 235U. Traces of curium may occur naturally in uranium minerals due to neutron capture and beta decay, though this has not been confirmed. Traces of 247Cm are also probably brought to Earth in cosmic ray
Cosmic rays are high-energy particles or clusters of particles (primarily represented by protons or atomic nuclei) that move through space at nearly the speed of light. They originate from the Sun, from outside of the Solar System in our own ...
s, but again this has not been confirmed.
Curium is made artificially in small amounts for research purposes. It also occurs as one of the waste products in spent nuclear fuel
Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor (usually at a nuclear power plant). It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor and ...
. Curium is present in nature in some areas used for nuclear weapons testing
Nuclear weapons tests are experiments carried out to determine nuclear weapons' effectiveness, Nuclear weapon yield, yield, and explosive capability. Testing nuclear weapons offers practical information about how the weapons function, how detona ...
.Curium(in German) Analysis of the debris at the test site of the
United States
The United States of America (U.S.A. or USA), commonly known as the United States (U.S. or US) or America, is a country primarily located in North America. It consists of 50 states, a federal district, five major unincorporated territorie ...
' first thermonuclear weapon
A thermonuclear weapon, fusion weapon or hydrogen bomb (H bomb) is a second-generation nuclear weapon design. Its greater sophistication affords it vastly greater destructive power than first-generation nuclear bombs, a more compact size, a lowe ...
, Ivy Mike
Ivy Mike was the codename given to the first full-scale test of a thermonuclear device, in which part of the explosive yield comes from nuclear fusion.
Ivy Mike was detonated on November 1, 1952, by the United States on the island of Elugelab in ...
, (1 November 1952, Enewetak Atoll), besides einsteinium
Einsteinium is a synthetic element with the symbol Es and atomic number 99. Einsteinium is a member of the actinide series and it is the seventh transuranium element. It was named in honor of Albert Einstein.
Einsteinium was discovered as a com ...
, fermium
Fermium is a synthetic element with the symbol Fm and atomic number 100. It is an actinide and the heaviest element that can be formed by neutron bombardment of lighter elements, and hence the last element that can be prepared in macroscopic qua ...
, plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibi ...
and americium
Americium is a synthetic radioactive chemical element with the symbol Am and atomic number 95. It is a transuranic member of the actinide series, in the periodic table located under the lanthanide element europium, and thus by analogy was na ...
also revealed isotopes of berkelium, californium and curium, in particular 245Cm, 246Cm and smaller quantities of 247Cm, 248Cm and 249Cm.
Atmospheric curium compounds are poorly soluble in common solvents and mostly adhere to soil particles. Soil analysis revealed about 4,000 times higher concentration of curium at the sandy soil particles than in water present in the soil pores. An even higher ratio of about 18,000 was measured in loam
Loam (in geology and soil science) is soil composed mostly of sand (particle size > ), silt (particle size > ), and a smaller amount of clay (particle size < ). By weight, its mineral composition is about 40–40–20% concentration of sand–sil ...
soils.
The transuranium element
The transuranium elements (also known as transuranic elements) are the chemical elements with atomic numbers greater than 92, which is the atomic number of uranium. All of these elements are unstable and decay radioactively into other elements. ...
s from americium to fermium, including curium, occurred naturally in the natural nuclear fission reactor
A natural nuclear fission reactor is a uranium deposit where self-sustaining nuclear chain reactions occur. The conditions under which a natural nuclear reactor could exist had been predicted in 1956 by Japanese American chemist Paul Kuroda. ...
at Oklo
Oklo is a region near the town of Franceville, in the Haut-Ogooué province of the Central African country of Gabon. Several natural nuclear fission reactors were discovered in the uranium mines in the region in 1972.
History
Gabon was a Fren ...
, but no longer do so.
Curium, and other non-primordial actinides, have also been detected in the spectrum of Przybylski's Star.
Synthesis
Isotope preparation
Curium is made in small amounts innuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat fr ...
s, and by now only kilograms of 242Cm and 244Cm have been accumulated, and grams or even milligrams for heavier isotopes. Hence the high price of curium, which has been quoted at 160–185 USD
The United States dollar (symbol: $; code: USD; also abbreviated US$ or U.S. Dollar, to distinguish it from other dollar-denominated currencies; referred to as the dollar, U.S. dollar, American dollar, or colloquially buck) is the official ...
per milligram, with a more recent estimate at US$2,000/g for 242Cm and US$170/g for 244Cm. In nuclear reactors, curium is formed from 238U in a series of nuclear reactions. In the first chain, 238U captures a neutron and converts into 239U, which via β− decay transforms into 239Np and 239Pu.
Further neutron capture followed by β−-decay gives americium
Americium is a synthetic radioactive chemical element with the symbol Am and atomic number 95. It is a transuranic member of the actinide series, in the periodic table located under the lanthanide element europium, and thus by analogy was na ...
(241Am) which further becomes 242Cm:
For research purposes, curium is obtained by irradiating not uranium but plutonium, which is available in large amounts from spent nuclear fuel. A much higher neutron flux is used for the irradiation that results in a different reaction chain and formation of 244Cm:Morss, L. R.; Edelstein, N. M. and Fugere, J. (eds): ''The Chemistry of the Actinide Elements and transactinides'', volume 3, Springer-Verlag, Dordrecht 2006, .
Curium-244 alpha decays to 240Pu, but it also absorbs neutrons, hence a small amount of heavier curium isotopes. Of those, 247Cm and 248Cm are popular in scientific research due to their long half-lives. But the production rate of 247Cm in thermal neutron reactors is low because it is prone to fission due to thermal neutrons. Synthesis of 250Cm by neutron capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, ...
is unlikely due to the short half-life of the intermediate 249Cm (64 min), which β− decays to the berkelium
Berkelium is a transuranic radioactive chemical element with the symbol Bk and atomic number 97. It is a member of the actinide and transuranium element series. It is named after the city of Berkeley, California, the location of the Lawrence Ber ...
isotope 249Bk.
The above cascade of (n,γ) reactions gives a mix of different curium isotopes. Their post-synthesis separation is cumbersome, so a selective synthesis is desired. Curium-248 is favored for research purposes due to its long half-life. The most efficient way to prepare this isotope is by α-decay of the californium
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding ...
isotope 252Cf, which is available in relatively large amounts due to its long half-life (2.65 years). About 35–50 mg of 248Cm is produced thus, per year. The associated reaction produces 248Cm with isotopic purity of 97%.
Another isotope, 245Cm, can be obtained for research, from α-decay of 249Cf; the latter isotope is produced in small amounts from β−-decay of 249 Bk.
Metal preparation
 Most synthesis routines yield a mix of actinide isotopes as
Most synthesis routines yield a mix of actinide isotopes as oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the E ...
s, from which a given isotope of curium needs to be separated. An example procedure could be to dissolve spent reactor fuel (e.g. MOX fuel
Mixed oxide fuel, commonly referred to as MOX fuel, is nuclear fuel that contains more than one oxide of fissile material, usually consisting of plutonium blended with natural uranium, reprocessed uranium, or depleted uranium. MOX fuel is an al ...
) in nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitri ...
, and remove the bulk of the uranium and plutonium using a PUREX
PUREX (plutonium uranium reduction extraction) is a chemical method used to purify fuel for nuclear reactors or nuclear weapons. PUREX is the ''de facto'' standard aqueous nuclear reprocessing method for the recovery of uranium and plutonium ...
(Plutonium – URanium EXtraction) type extraction with tributyl phosphate
Tributyl phosphate, known commonly as TBP, is an organophosphorus compound with the chemical formula (CH3CH2CH2CH2O)3PO. This colourless, odorless liquid finds some applications as an extractant and a plasticizer. It is an ester of phosphoric ac ...
in a hydrocarbon. The lanthanides and the remaining actinides are then separated from the aqueous residue (raffinate In chemical separation terminology, the raffinate (from French ''raffiner'', to refine) is a product which has had a component or components removed. The product having the removed materials is referred to as the extract. For example, in solvent ex ...
) by a diamide-based extraction to give, after stripping, a mixture of trivalent actinides and lanthanides. A curium compound is then selectively extracted using multi-step chromatographic
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system (a ...
and centrifugation techniques with an appropriate reagent. ''Bis''-triazinyl bipyridine complex has been recently proposed as such reagent which is highly selective to curium. Separation of curium from the very chemically similar americium can also be done by treating a slurry of their hydroxides in aqueous sodium bicarbonate with ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lo ...
at elevated temperature. Both americium and curium are present in solutions mostly in the +3 valence state; americium oxidizes to soluble Am(IV) complexes, but curium stays unchanged and so can be isolated by repeated centrifugation.
Metallic curium is obtained by reduction of its compounds. Initially, curium(III) fluoride was used for this purpose. The reaction was done in an environment free of water and oxygen, in an apparatus made of tantalum
Tantalum is a chemical element with the symbol Ta and atomic number 73. Previously known as ''tantalium'', it is named after Tantalus, a villain in Greek mythology. Tantalum is a very hard, ductile, lustrous, blue-gray transition metal that is ...
and tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isolat ...
, using elemental barium or lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid el ...
as reducing agents.
:
Another possibility is reduction of curium(IV) oxide using a magnesium-zinc alloy in a melt of magnesium chloride
Magnesium chloride is the family of inorganic compounds with the formula , where x can range from 0 to 12. These salts are colorless or white solids that are highly soluble in water. These compounds and their solutions, both of which occur in natu ...
and magnesium fluoride
Magnesium fluoride is an inorganic compound with the formula MgF2. The compound is a white crystalline salt and is transparent over a wide range of wavelengths, with commercial uses in optics that are also used in space telescopes. It occurs natur ...
.
Compounds and reactions
Oxides
Curium readily reacts with oxygen forming mostly Cm2O3 and CmO2 oxides, but the divalent oxide CmO is also known.Holleman, p. 1972 Black CmO2 can be obtained by burning curiumoxalate
Oxalate (IUPAC: ethanedioate) is an anion with the formula C2O42−. This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (Na2C2O4), and several esters such as dimethyl o ...
(), nitrate (), or hydroxide in pure oxygen.Greenwood, p. 1268 Upon heating to 600–650 °C in vacuum (about 0.01 Pa), it transforms into the whitish Cm2O3:
: Delta T
Delta commonly refers to:
* Delta (letter) (Δ or δ), a letter of the Greek alphabet
* River delta, at a river mouth
* D (NATO phonetic alphabet: "Delta")
* Delta Air Lines, US
* Delta variant of SARS-CoV-2 that causes COVID-19
Delta may also r ...
2Cm2O3 + O2hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
:
: Halides
The colorless curium(III) fluoride (CmF3) can be made by adding fluoride ions into curium(III)-containing solutions. The brown tetravalent curium(IV) fluoride (CmF4) on the other hand is only obtained by reacting curium(III) fluoride with molecularfluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reacti ...
:
:
A series of ternary fluorides are known of the form A7Cm6F31 (A = alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
).
The colorless curium(III) chloride (CmCl3) is made by reacting curium hydroxide (Cm(OH)3) with anhydrous hydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride ga ...
gas. It can be further turned into other halides such as curium(III) bromide (colorless to light green) and curium(III) iodide (colorless), by reacting it with the ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
salt of the corresponding halide at temperatures of ~400–450°C:
:
Or, one can heat curium oxide to ~600°C with the corresponding acid (such as hydrobromic for curium bromide). Vapor phase hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
of curium(III) chloride gives curium oxychloride:
:
Chalcogenides and pnictides
Sulfides, selenides and tellurides of curium have been obtained by treating curium with gaseoussulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
, selenium
Selenium is a chemical element with the symbol Se and atomic number 34. It is a nonmetal (more rarely considered a metalloid) with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, ...
or tellurium
Tellurium is a chemical element with the symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally fou ...
in vacuum at elevated temperature. Curium pnictides
A pnictogen ( or ; from grc, wikt:πνίγω, πνῑ́γω "to choke" and wikt:-gen#English, -gen, "generator") is any of the chemical elements in group (periodic table), group 15 of the periodic table. Group 15 is also known as the nitro ...
of the type CmX are known for nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
, phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ear ...
, arsenic
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, but ...
and antimony
Antimony is a chemical element with the symbol Sb (from la, stibium) and atomic number 51. A lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient time ...
. They can be prepared by reacting either curium(III) hydride (CmH3) or metallic curium with these elements at elevated temperature.Lumetta, G. J.; Thompson, M. C.; Penneman, R. A.; Eller, P. GCurium
, Chapter Nine in ''Radioanalytical Chemistry'', Springer, 2004, pp. 1420–1421. ,
Organocurium compounds and biological aspects
 Organometallic complexes analogous to
Organometallic complexes analogous to uranocene
Uranocene, U(C8H8)2, is an organouranium compound composed of a uranium atom sandwiched between two cyclooctatetraenide rings. It was one of the first organoactinide compounds to be synthesized. It is a green air-sensitive solid that dissolves in ...
are known also for other actinides, such as thorium, protactinium, neptunium, plutonium and americium. Molecular orbital theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century.
In molecular orbital theory, electrons in a molecule ...
predicts a stable "curocene" complex (η8-C8H8)2Cm, but it has not been reported experimentally yet.
Formation of the complexes of the type (BTP = 2,6-di(1,2,4-triazin-3-yl)pyridine), in solutions containing n-C3H7-BTP and Cm3+ ions has been confirmed by EXAFS
Extended X-ray absorption fine structure (EXAFS), along with X-ray absorption near edge structure (XANES), is a subset of X-ray absorption spectroscopy ( XAS). Like other absorption spectroscopies, XAS techniques follow Beer's law. The X-ray ...
. Some of these BTP-type complexes selectively interact with curium and thus are useful for separating it from lanthanides and another actinides. Dissolved Cm3+ ions bind with many organic compounds, such as hydroxamic acid
A hydroxamic acid is a class of organic compounds bearing the functional group RC(O)N(OH)R', with R and R' as organic residues and CO as a carbonyl group. They are amides (RC(O)NHR') wherein the NH center has an OH substitution. They are often us ...
, urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid.
Urea serves an important r ...
, fluorescein
Fluorescein is an organic compound and dye based on the xanthene tricyclic structural motif, formally belonging to triarylmethine dyes family. It is available as a dark orange/red powder slightly soluble in water and alcohol. It is widely used ...
and adenosine triphosphate
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of ...
. Many of these compounds are related to biological activity of various microorganism
A microorganism, or microbe,, ''mikros'', "small") and ''organism'' from the el, ὀργανισμός, ''organismós'', "organism"). It is usually written as a single word but is sometimes hyphenated (''micro-organism''), especially in olde ...
s. The resulting complexes show strong yellow-orange emission under UV light excitation, which is convenient not only for their detection, but also for studying interactions between the Cm3+ ion and the ligands via changes in the half-life (of the order ~0.1 ms) and spectrum of the fluorescence.
Curium has no biological significance. There are a few reports on biosorption Biosorption is a physiochemical process that occurs naturally in certain biomass which allows it to passively concentrate and bind contaminants onto its cellular structure. Biosorption can be defined as the ability of biological materials to accumu ...
of Cm3+ by bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among ...
and archaea
Archaea ( ; singular archaeon ) is a domain of single-celled organisms. These microorganisms lack cell nuclei and are therefore prokaryotes. Archaea were initially classified as bacteria, receiving the name archaebacteria (in the Archaebac ...
, but no evidence for incorporation of curium into them.
Applications
Radionuclides
Curium is one of the most radioactive isolable elements. Its two most common isotopes 242Cm and 244Cm are strong alpha emitters (energy 6 MeV); they have fairly short half-lives, 162.8 days and 18.1 years, and give as much as 120 W/g and 3 W/g of heat, respectively.Binder, Harry H.: ''Lexikon der chemischen Elemente'', S. Hirzel Verlag, Stuttgart 1999, , pp. 174–178. Therefore, curium can be used in its common oxide form inradioisotope thermoelectric generator
A radioisotope thermoelectric generator (RTG, RITEG), sometimes referred to as a radioisotope power system (RPS), is a type of nuclear battery that uses an array of thermocouples to convert the heat released by the decay of a suitable radioacti ...
s like those in spacecraft. This application has been studied for the 244Cm isotope, while 242Cm was abandoned due to its prohibitive price, around 2000 USD/g. 243Cm with a ~30-year half-life and good energy yield of ~1.6 W/g could be a suitable fuel, but it gives significant amounts of harmful gamma
Gamma (uppercase , lowercase ; ''gámma'') is the third letter of the Greek alphabet. In the system of Greek numerals it has a value of 3. In Ancient Greek, the letter gamma represented a voiced velar stop . In Modern Greek, this letter re ...
and beta
Beta (, ; uppercase , lowercase , or cursive ; grc, βῆτα, bē̂ta or ell, βήτα, víta) is the second letter of the Greek alphabet. In the system of Greek numerals, it has a value of 2. In Modern Greek, it represents the voiced labiod ...
rays from radioactive decay products. As an α-emitter, 244Cm needs much less radiation shielding, but it has a high spontaneous fission rate, and thus a lot of neutron and gamma radiation. Compared to a competing thermoelectric generator isotope such as 238Pu, 244Cm emits 500 times more neutrons, and its higher gamma emission requires a shield that is 20 times thicker— of lead for a 1 kW source, compared to for 238Pu. Therefore, this use of curium is currently considered impractical.Basic elements of static RTGs, G.L. Kulcinski, NEEP 602 Course Notes (Spring 2000), Nuclear Power in Space, University of Wisconsin Fusion Technology Institute (see last page) A more promising use of 242Cm is for making 238Pu, a better radioisotope for thermoelectric generators such as in heart pacemakers. The alternate routes to 238Pu use the (n,γ) reaction of 237Np, or
deuteron
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two stable isotopes of hydrogen (the other being protium, or hydrogen-1). The nucleus of a deuterium atom, called a deuteron, contains one proton and one n ...
bombardment of uranium, though both reactions always produce 236Pu as an undesired by-product since the latter decays to 232U with strong gamma emission. Curium is a common starting material for making higher transuranic
The transuranium elements (also known as transuranic elements) are the chemical elements with atomic numbers greater than 92, which is the atomic number of uranium. All of these elements are unstable and decay radioactively into other elements. ...
and superheavy element
Superheavy elements, also known as transactinide elements, transactinides, or super-heavy elements, are the chemical elements with atomic number greater than 103. The superheavy elements are those beyond the actinides in the periodic table; the l ...
s. Thus, bombarding 248Cm with neon (22Ne), magnesium (26Mg), or calcium ( 48Ca) yields isotopes of seaborgium
Seaborgium is a synthetic chemical element with the symbol Sg and atomic number 106. It is named after the American nuclear chemist Glenn T. Seaborg. As a synthetic element, it can be created in a laboratory but is not found in nature. It is al ...
(265Sg), hassium
Hassium is a chemical element with the symbol Hs and the atomic number 108. Hassium is highly radioactive; its most stable known isotopes have half-lives of approximately ten seconds. One of its isotopes, 270Hs, has magic numbers of both protons ...
(269Hs and 270Hs), and livermorium
Livermorium is a synthetic chemical element with the symbol Lv and has an atomic number of 116. It is an extremely radioactive element that has only been created in a laboratory setting and has not been observed in nature. The element is named afte ...
(292Lv, 293Lv, and possibly 294Lv).Holleman, pp. 1980–1981. Californium was discovered when a microgram-sized target of curium-242 was irradiated with 35 MeV alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be pr ...
s using the cyclotron at Berkeley:
: + → +
Only about 5,000 atoms of californium were produced in this experiment.
The odd-mass curium isotopes 243Cm, 245Cm, and 247Cm are all highly fissile
In nuclear engineering, fissile material is material capable of sustaining a nuclear fission chain reaction. By definition, fissile material can sustain a chain reaction with neutrons of thermal energy. The predominant neutron energy may be typ ...
and can release additional energy in a thermal spectrum nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat fr ...
. All curium isotopes are fissionable in fast-neutron reactors. This is one of the motives for minor actinide
The minor actinides are the actinide elements in used nuclear fuel other than uranium and plutonium, which are termed the major actinides. The minor actinides include neptunium (element 93), americium (element 95), curium (element 96), berkeliu ...
separation and transmutation in the nuclear fuel cycle
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the ''front end'', which are the preparation of the fuel, steps in the ''service period'' in w ...
, helping to reduce the long-term radiotoxicity of used, or spent nuclear fuel
Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor (usually at a nuclear power plant). It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor and ...
.

X-ray spectrometer
The most practical application of 244Cm—though rather limited in total volume—is as α-particle source inalpha particle X-ray spectrometer
:''APXS is also an abbreviation for APache eXtenSion tool, an extension for Apache web servers.''
An alpha particle X-ray spectrometer (APXS) is a spectrometer that analyses the chemical element composition of a sample from scattered alpha part ...
s (APXS). These instruments were installed on the Sojourner
A sojourner is a person who resides temporarily in a place.
Sojourner may also refer to:
* Sojourner Truth (1797–1883), abolitionist and women's rights activist
* Albert Sojourner (1872–1951), member of the Mississippi House of Representative ...
, Mars
Mars is the fourth planet from the Sun and the second-smallest planet in the Solar System, only being larger than Mercury (planet), Mercury. In the English language, Mars is named for the Mars (mythology), Roman god of war. Mars is a terr ...
, Mars 96
Mars 96 (sometimes called Mars-8) was a failed Mars mission launched in 1996 to investigate Mars by the Russian Space Forces and not directly related to the Soviet Mars probe program of the same name. After failure of the second fourth-stage bu ...
, Mars Exploration Rover
NASA's Mars Exploration Rover (MER) mission was a robotic space mission involving two Mars rovers, ''Spirit (rover), Spirit'' and ''Opportunity (rover), Opportunity'', exploring the planet Mars. It began in 2003 with the launch of the two rover ...
s and Philae comet lander, as well as the Mars Science Laboratory
Mars Science Laboratory (MSL) is a robotic space probe mission to Mars launched by NASA on November 26, 2011, which successfully landed ''Curiosity'', a Mars rover, in Gale Crater on August 6, 2012. The overall objectives include investigati ...
to analyze the composition and structure of the rocks on the surface of planet Mars
Mars is the fourth planet from the Sun and the second-smallest planet in the Solar System, only being larger than Mercury (planet), Mercury. In the English language, Mars is named for the Mars (mythology), Roman god of war. Mars is a terr ...
. APXS was also used in the Surveyor 5–7 moon probes but with a 242Cm source.Human Health Fact Sheet on Curium, Los Alamos National Laboratory An elaborate APXS setup has a sensor head containing six curium sources with a total decay rate of several tens of millicuries (roughly one
gigabecquerel
The becquerel (; symbol: Bq) is the unit of Radioactive decay, radioactivity in the International System of Units (SI). One becquerel is defined as the activity (radioactivity), activity of a quantity of radioactive material in which one atomic n ...
). The sources are collimated on a sample, and the energy spectra of the alpha particles and protons scattered from the sample are analyzed (proton analysis is done only in some spectrometers). These spectra contain quantitative information on all major elements in the sample except for hydrogen, helium and lithium.
Safety
Due to its radioactivity, curium and its compounds must be handled in appropriate labs under special arrangements. While curium itself mostly emits α-particles which are absorbed by thin layers of common materials, some of its decay products emit significant fractions of beta and gamma rays, which require a more elaborate protection. If consumed, curium is excreted within a few days and only 0.05% is absorbed in the blood. From there, ~45% goes to theliver
The liver is a major Organ (anatomy), organ only found in vertebrates which performs many essential biological functions such as detoxification of the organism, and the Protein biosynthesis, synthesis of proteins and biochemicals necessary for ...
, 45% to the bones, and the remaining 10% is excreted. In bone, curium accumulates on the inside of the interfaces to the bone marrow
Bone marrow is a semi-solid tissue found within the spongy (also known as cancellous) portions of bones. In birds and mammals, bone marrow is the primary site of new blood cell production (or haematopoiesis). It is composed of hematopoietic ce ...
and does not significantly redistribute with time; its radiation destroys bone marrow and thus stops red blood cell
Red blood cells (RBCs), also referred to as red cells, red blood corpuscles (in humans or other animals not having nucleus in red blood cells), haematids, erythroid cells or erythrocytes (from Greek ''erythros'' for "red" and ''kytos'' for "holl ...
creation. The biological half-life
Biological half-life (also known as elimination half-life, pharmacologic half-life) is the time taken for concentration of a biological substance (such as a medication) to decrease from its maximum concentration ( Cmax) to half of Cmax in the bl ...
of curium is about 20 years in the liver and 50 years in the bones. Curium is absorbed in the body much more strongly via inhalation, and the allowed total dose of 244Cm in soluble form is 0.3 μ Ci. Intravenous injection of 242Cm- and 244Cm-containing solutions to rats increased the incidence of bone tumor
A bone tumor is an abnormal growth of tissue in bone, traditionally classified as noncancerous (benign) or cancerous (malignant). Cancerous bone tumors usually originate from a cancer in another part of the body such as from lung, breast, thyroi ...
, and inhalation promoted lung
The lungs are the primary organs of the respiratory system in humans and most other animals, including some snails and a small number of fish. In mammals and most other vertebrates, two lungs are located near the backbone on either side of t ...
and liver cancer
Liver cancer (also known as hepatic cancer, primary hepatic cancer, or primary hepatic malignancy) is cancer that starts in the liver. Liver cancer can be primary (starts in liver) or secondary (meaning cancer which has spread from elsewhere to th ...
.
Curium isotopes are inevitably present in spent nuclear fuel (about 20 g/tonne). The isotopes 245Cm–248Cm have decay times of thousands of years and must be removed to neutralize the fuel for disposal.Baetslé, L. HApplication of Partitioning/Transmutation of Radioactive Materials in Radioactive Waste Management
, Nuclear Research Centre of Belgium Sck/Cen, Mol, Belgium, September 2001. Such a procedure involves several steps, where curium is first separated and then converted by neutron bombardment in special reactors to short-lived nuclides. This procedure,
nuclear transmutation
Nuclear transmutation is the conversion of one chemical element or an isotope into another chemical element. Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus of an atom is changed.
A transmutatio ...
, while well documented for other elements, is still being developed for curium.
References
Bibliography
* * Holleman, Arnold F. and Wiberg, Nils '' Lehrbuch der Anorganischen Chemie'', 102 Edition, de Gruyter, Berlin 2007, . * Penneman, R. A. and Keenan T. KThe radiochemistry of americium and curium
University of California, Los Alamos, California, 1960
External links
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics i ...
'' (University of Nottingham)
NLM Hazardous Substances Databank – Curium, Radioactive
{{Marie & Pierre Curie Chemical elements Chemical elements with double hexagonal close-packed structure Actinides American inventions Synthetic elements Marie Curie Pierre Curie