Cubic Harmonic on:
[Wikipedia]
[Google]
[Amazon]
 In fields like computational chemistry and
In fields like computational chemistry and
 First of all, the cubic harmonics are ''real functions'', while spherical harmonics are ''
First of all, the cubic harmonics are ''real functions'', while spherical harmonics are ''
 In fields like computational chemistry and
In fields like computational chemistry and solid-state
Solid state, or solid matter, is one of the four fundamental states of matter.
Solid state may also refer to:
Electronics
* Solid-state electronics, circuits built of solid materials
* Solid state ionics, study of ionic conductors and their use ...
and condensed matter
Condensed matter physics is the field of physics that deals with the macroscopic and microscopic physical properties of matter, especially the solid and liquid phases which arise from electromagnetic forces between atoms. More generally, the su ...
physics the so-called atomic orbitals
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any sp ...
, or spin-orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any spe ...
s, as they appear in textbooks
on quantum physics, are often partially replaced by cubic harmonics for a number of reasons. These harmonics are usually named tesseral harmonics in the field of condensed matter physics in which the name kubic harmonics rather refers to the irreducible representations in the cubic point-group.
Introduction
The hydrogen-like atomic orbitals with principal quantum number and angular momentum quantum number are often expressed as : in which the is the radial part of the wave function and is the angular dependent part. The are thespherical harmonics
In mathematics and physical science, spherical harmonics are special functions defined on the surface of a sphere. They are often employed in solving partial differential equations in many scientific fields.
Since the spherical harmonics form ...
, which are solutions of the angular momentum
In physics, angular momentum (rarely, moment of momentum or rotational momentum) is the rotational analog of linear momentum. It is an important physical quantity because it is a conserved quantity—the total angular momentum of a closed syst ...
operator. The spherical harmonics are representations of functions of the full rotation group SO(3)
with rotational symmetry. In many fields of physics and chemistry these spherical harmonics are replaced by cubic harmonics because the rotational symmetry of the atom and its environment are distorted or because cubic harmonics offer computational benefits.
Symmetry and coordinate system
In many cases, especially in chemistry andsolid-state
Solid state, or solid matter, is one of the four fundamental states of matter.
Solid state may also refer to:
Electronics
* Solid-state electronics, circuits built of solid materials
* Solid state ionics, study of ionic conductors and their use ...
and condensed-matter physics
Condensed matter physics is the field of physics that deals with the macroscopic and microscopic physical properties of matter, especially the solid and liquid phases which arise from electromagnetic forces between atoms. More generally, the su ...
, the system under investigation doesn't have rotational symmetry. Often it has some kind of lower symmetry, with a special point group representation, or it has no spatial symmetry at all. Biological and biochemical systems, like amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
s and enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
s often belong to low molecular symmetry point groups. The solid crystals of the elements often belong to the space group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it uncha ...
s and point groups with high symmetry. (Cubic harmonics representations are often listed and referenced in point group tables.) The system has at least a fixed orientation in three-dimensional Euclidean space
Euclidean space is the fundamental space of geometry, intended to represent physical space. Originally, that is, in Euclid's ''Elements'', it was the three-dimensional space of Euclidean geometry, but in modern mathematics there are Euclidean ...
. Therefore, the coordinate system that is used in such cases is most often a Cartesian coordinate system instead of a spherical coordinate system. In a Cartesian coordinate system the atomic orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in an ...
s are often expressed as
:
with the cubic harmonics,
, as a basis set. LCAO and MO calculations in computational chemistry or tight binding
In solid-state physics, the tight-binding model (or TB model) is an approach to the calculation of electronic band structure using an approximate set of wave functions based upon superposition of wave functions for isolated atoms located at eac ...
calculations in solid-state physics use cubic harmonics as an atomic orbital basis. The indices ''lc'' are denoting some kind of Cartesian representation.
Basis transformations
For therepresentations
''Representations'' is an interdisciplinary journal in the humanities published quarterly by the University of California Press. The journal was established in 1983 and is the founding publication of the New Historicism movement of the 1980s. It ...
of the spherical harmonics a spherical coordinate system is chosen with a principal axis in the z-direction. For the cubic harmonics this axis is also the most convenient choice. For states of higher angular momentum quantum number and a higher dimension of the number of possible rotations or basis transformations in Hilbert space grows and so does the number of possible orthogonal representations that can be constructed on the basis of the -dimensional spherical harmonics basis set. There is more freedom to choose a representation that fits the point group symmetry of the problem. The cubic representations that are listed in the table are a result of the transformations, which are 45° 2D rotations and a 90° rotation to the real axis if necessary, like
:
:
:
A substantial number of the spherical harmonics are listed in the Table of spherical harmonics This is a table of orthonormalized spherical harmonics that employ the Condon-Shortley phase up to degree \ell = 10. Some of these formulas are expressed in terms of the Cartesian expansion of the spherical harmonics into polynomials in ''x'', ''y'' ...
.
Computational benefits
complex functions
Complex analysis, traditionally known as the theory of functions of a complex variable, is the branch of mathematical analysis that investigates functions of complex numbers. It is helpful in many branches of mathematics, including algebrai ...
''. The complex numbers are two-dimensional with a real part and an imaginary part. Complex numbers offer very handsome and effective tools to tackle mathematical problems analytically but they are not very effective when they are used for numerical calculations. Skipping the imaginary part saves half the calculational effort in summations, a factor of four in multiplications and often factors of eight or even more when it comes to computations involving matrices.
The cubic harmonics often fit the symmetry of the potential or surrounding of an atom. A common surrounding of atoms in solids and chemical complexes is an octahedral surrounding with an octahedral cubic point group symmetry. The representations of the cubic harmonics often have a high symmetry and multiplicity so operations like integrations can be reduced to a limited, or irreducible, part of the domain of the function that has to be evaluated. A problem with the 48-fold octahedral Oh symmetry can be calculated much faster if one limits a calculation, like an integration, to the irreducible part of the domain of the function.
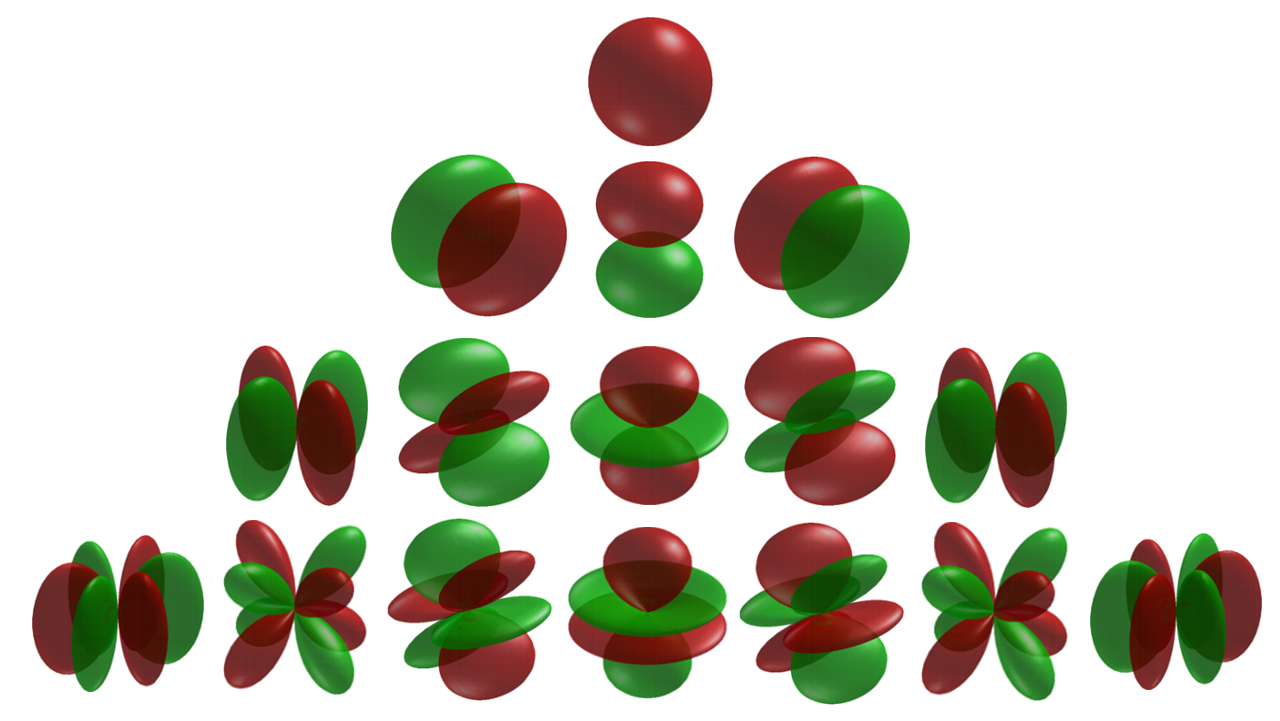
Table of cubic harmonics
The s-orbitals
The s-orbitals only have a radial part. : :The p-orbitals
The three p-orbitals areatomic orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in an ...
s with an angular momentum quantum number ℓ = 1. The cubic harmonic expression of the p-orbitals
:
:
:
with
:
The d-orbitals
The five d-orbitals areatomic orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in an ...
s with an angular momentum quantum number ℓ = 2. The angular part of the d-orbitals are often expressed like
:
The angular part of the d-orbitals are the cubic harmonics
:
:
:
:
:
with
:
The f-orbitals
The seven f-orbitals areatomic orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in an ...
s with an angular momentum quantum number ℓ = 3. often expressed like
:
The angular part of the f-orbitals are the cubic harmonics . In many cases different linear combinations of spherical harmonics are chosen to construct a cubic f-orbital basis set.
:
:
:
:
:
:
:
with
:
See also
*Atomic orbitals
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any sp ...
* Atomic physics
* Spherical harmonics
In mathematics and physical science, spherical harmonics are special functions defined on the surface of a sphere. They are often employed in solving partial differential equations in many scientific fields.
Since the spherical harmonics form ...
* Spherical coordinate system
* Cartesian coordinate system
* Euclidean space
Euclidean space is the fundamental space of geometry, intended to represent physical space. Originally, that is, in Euclid's ''Elements'', it was the three-dimensional space of Euclidean geometry, but in modern mathematics there are Euclidean ...
* Hilbert space
* Basis set (chemistry)
In theoretical and computational chemistry, a basis set is a set of functions (called basis functions) that is used to represent the electronic wave function in the Hartree–Fock method or density-functional theory in order to turn the parti ...
* Basis (linear algebra)
In mathematics, a set of vectors in a vector space is called a basis if every element of may be written in a unique way as a finite linear combination of elements of . The coefficients of this linear combination are referred to as component ...
* Coordinate vector
* LCAO method
* Tight binding method
References
{{atomic models Molecular physics Quantum chemistry Electronic structure methods Physical chemistry Euclidean symmetries