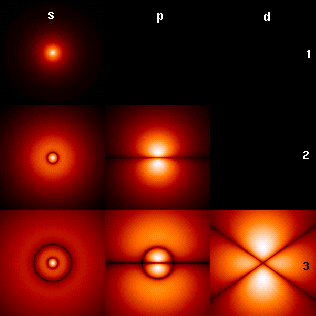
|
Spin-orbital
In atomic theory and quantum mechanics, an atomic orbital is a Function (mathematics), function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the Atomic nucleus, atom's nucleus. The term ''atomic orbital'' may also refer to the physical region or space where the electron can be calculated to be present, as predicted by the particular mathematical form of the orbital. Each orbital in an atom is characterized by a set of values of the three quantum numbers , , and , which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (magnetic quantum number). Alternative to the magnetic quantum number, the orbitals are often labeled by the associated Spherical harmonics#Harmonic polynomial representation, harmonic polynomials (e.g., ''xy'', ). Each such orbital can be occupied by a maximum o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neon Orbitals
Neon is a chemical element with the symbol Ne and atomic number 10. It is a noble gas. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with about two-thirds the density of air. It was discovered (along with krypton and xenon) in 1898 as one of the three residual rare inert elements remaining in dry air, after nitrogen, oxygen, argon and carbon dioxide were removed. Neon was the second of these three rare gases to be discovered and was immediately recognized as a new element from its bright red emission spectrum. The name neon is derived from the Greek word, , neuter singular form of (), meaning 'new'. Neon is chemically inert, and no uncharged neon compounds are known. The compounds of neon currently known include ionic molecules, molecules held together by van der Waals forces and clathrates. During cosmic nucleogenesis of the elements, large amounts of neon are built up from the alpha-capture fusion process in stars. Although neon is a very c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectral Line
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from emission or absorption of light in a narrow frequency range, compared with the nearby frequencies. Spectral lines are often used to identify atoms and molecules. These "fingerprints" can be compared to the previously collected ones of atoms and molecules, and are thus used to identify the atomic and molecular components of stars and planets, which would otherwise be impossible. Types of line spectra Spectral lines are the result of interaction between a quantum system (usually atoms, but sometimes molecules or atomic nuclei) and a single photon. When a photon has about the right amount of energy (which is connected to its frequency) to allow a change in the energy state of the system (in the case of an atom this is usually an electron changing orbitals), the photon is absorbed. Then the energy will be spontaneously re-emitted, either as one photon at the same frequenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fundamental Frequency
The fundamental frequency, often referred to simply as the ''fundamental'', is defined as the lowest frequency of a periodic waveform. In music, the fundamental is the musical pitch of a note that is perceived as the lowest partial present. In terms of a superposition of sinusoids, the fundamental frequency is the lowest frequency sinusoidal in the sum of harmonically related frequencies, or the frequency of the difference between adjacent frequencies. In some contexts, the fundamental is usually abbreviated as 0, indicating the lowest frequency counting from zero. In other contexts, it is more common to abbreviate it as 1, the first harmonic. (The second harmonic is then 2 = 2⋅1, etc. In this context, the zeroth harmonic would be 0 Hz.) According to Benward's and Saker's ''Music: In Theory and Practice'': Explanation All sinusoidal and many non-sinusoidal waveforms repeat exactly over time – they are periodic. The period of a waveform is the smallest value of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
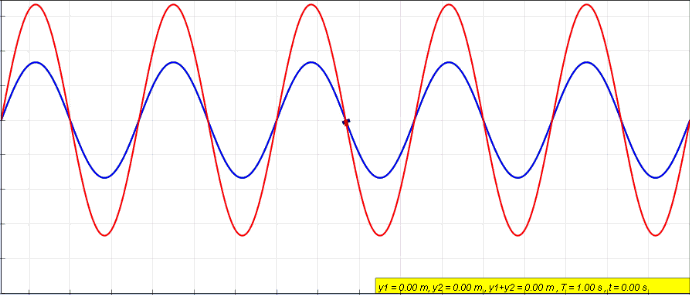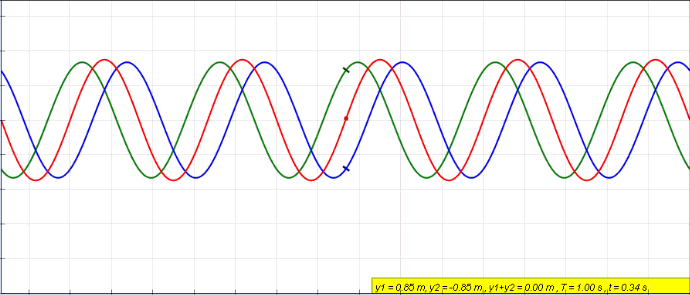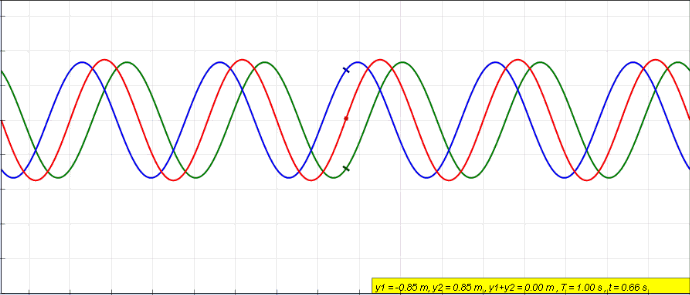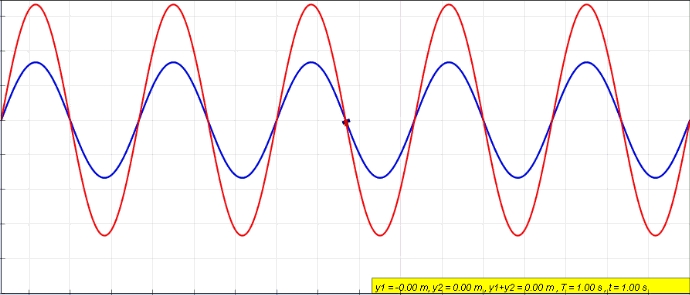
Standing Wave
In physics, a standing wave, also known as a stationary wave, is a wave that oscillates in time but whose peak amplitude profile does not move in space. The peak amplitude of the wave oscillations at any point in space is constant with respect to time, and the oscillations at different points throughout the wave are in phase. The locations at which the absolute value of the amplitude is minimum are called nodes, and the locations where the absolute value of the amplitude is maximum are called antinodes. Standing waves were first noticed by Michael Faraday in 1831. Faraday observed standing waves on the surface of a liquid in a vibrating container. Franz Melde coined the term "standing wave" (German: ''stehende Welle'' or ''Stehwelle'') around 1860 and demonstrated the phenomenon in his classic experiment with vibrating strings. This phenomenon can occur because the medium is moving in the direction opposite to the movement of the wave, or it can arise in a stationary medium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wave–particle Duality
Wave–particle duality is the concept in quantum mechanics that every particle or quantum entity may be described as either a particle or a wave. It expresses the inability of the classical concepts "particle" or "wave" to fully describe the behaviour of quantum-scale objects. As Albert Einstein wrote: Through the work of Max Planck, Albert Einstein, Louis de Broglie, Arthur Compton, Niels Bohr, Erwin Schrödinger and many others, current scientific theory holds that all particles exhibit a wave nature and vice versa. This phenomenon has been verified not only for elementary particles, but also for compound particles like atoms and even molecules. For macroscopic particles, because of their extremely short wavelengths, wave properties usually cannot be detected. Although the use of the wave–particle duality has worked well in physics, the meaning or interpretation has not been satisfactorily resolved; see interpretations of quantum mechanics. Bohr regarded the "duality ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Density Plots
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 yea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal. Chromium metal is valued for its high corrosion resistance and hardness. A major development in steel production was the discovery that steel could be made highly resistant to corrosion and discoloration by adding metallic chromium to form stainless steel. Stainless steel and chrome plating (electroplating with chromium) together comprise 85% of the commercial use. Chromium is also greatly valued as a metal that is able to be highly polished while resisting tarnishing. Polished chromium reflects almost 70% of the visible spectrum, and almost 90% of infrared light. The name of the element is derived from the Greek word χρῶμα, ''chrōma'', meaning color, because many chromium compounds are intensely colored. Industrial production of chromium proceeds from chromite ore (mostly FeCr2O4) to produce ferro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aufbau Principle
The aufbau principle , from the German ''Aufbauprinzip'' (building-up principle), also called the aufbau rule, states that in the ground state of an atom or ion, electrons fill subshells of the lowest available energy, then they fill subshells of higher energy. For example, the 1s subshell is filled before the 2s subshell is occupied. In this way, the electrons of an atom or ion form the most stable electron configuration possible. An example is the configuration for the phosphorus atom, meaning that the 1s subshell has 2 electrons, and so on. Electron behavior is elaborated by other principles of atomic physics, such as Hund's rule and the Pauli exclusion principle. Hund's rule asserts that if multiple orbitals of the same energy are available, electrons will occupy different orbitals singly and with the same spin before any are occupied doubly. If double occupation does occur, the Pauli exclusion principle requires that electrons that occupy the same orbital must have differ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Periodic Table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of chemistry. It is a graphic formulation of the periodic law, which states that the properties of the chemical elements exhibit an approximate periodic dependence on their atomic numbers. The table is divided into four roughly rectangular areas called blocks. The rows of the table are called periods, and the columns are called groups. Elements from the same group of the periodic table show similar chemical characteristics. Trends run through the periodic table, with nonmetallic character (keeping their own electrons) increasing from left to right across a period, and from down to up across a group, and metallic character (surrendering electrons to other atoms) increasing in the opposite direction. The underlying reason for these trends is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler substances by any chemical reaction. The number of protons in the nucleus is the defining property of an element, and is referred to as its atomic number (represented by the symbol ''Z'') – all atoms with the same atomic number are atoms of the same element. Almost all of the baryonic matter of the universe is composed of chemical elements (among rare exceptions are neutron stars). When different elements undergo chemical reactions, atoms are rearranged into new compounds held together by chemical bonds. Only a minority of elements, such as silver and gold, are found uncombined as relatively pure native element minerals. Nearly all other naturally occurring elements occur in the Earth as compounds or mixtures. Air is primarily a mixture o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the baryonic mass of the universe. In everyday life on Earth, isolated hydrogen atoms (called "atomic hydrogen") are extremely rare. Instead, a hydrogen atom tends to combine with other atoms in compounds, or with another hydrogen atom to form ordinary ( diatomic) hydrogen gas, H2. "Atomic hydrogen" and "hydrogen atom" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms). Atomic spectroscopy shows that there is a discrete infinite set of states in which a hydrogen (or any) atom can exist, contrary to the predictions of classical physics. Attempts to develop a theore ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fundamental Series
The fundamental series is a set of spectral lines in a set caused by transition between d and f orbitals in atoms. Originally the series was discovered in the infrared by Fowler and independently by Arno Bergmann. This resulted in the name Bergmann series used for such a set of lines in a spectrum. However the name was changed as Bergmann also discovered other series of lines. And other discoverers also established other such series. They became known as the fundamental series. Bergmann observed lithium at 5347 cm−1, sodium at 5416 cm−1 potassium at 6592 cm−1. Bergmann observed that the lines in the series in the caesium spectrum were double. His discovery was announced in ''Contributions to the Knowledge of the Infra-Red Emission Spectra of the Alkalies'', Jena 1907. Carl Runge called this series the "new series". He predicted that the lines of potassium and rubidium would be in pairs. He expressed the frequencies of the series lines by a formula and pred ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |





%2C_black_and_white.png)