|
Sabinene
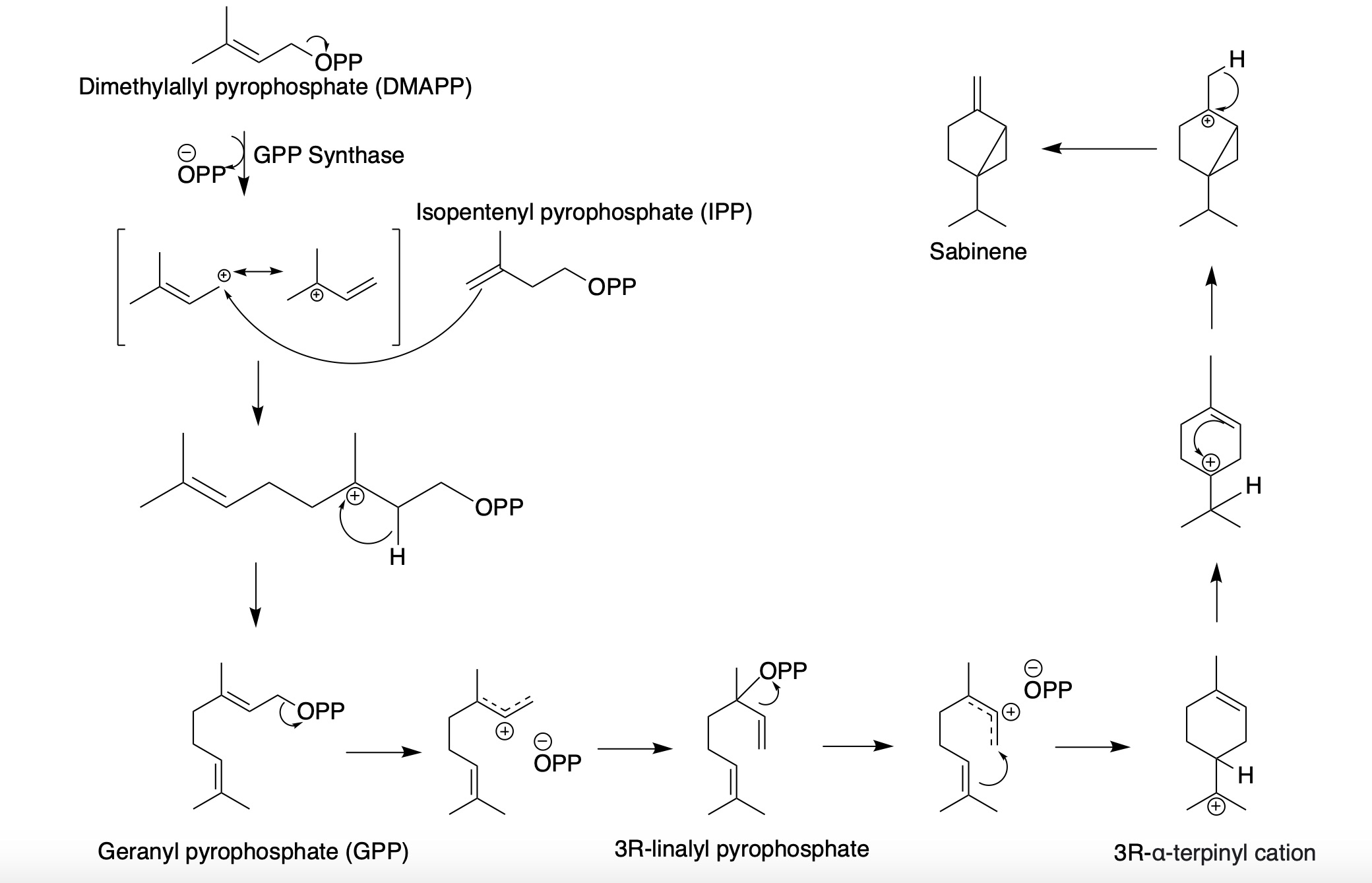
Sabinene is a natural bicyclic monoterpene with the molecular formula C10H16. It is isolated from the essential oils of a variety of plants including Marjoram, holm oak (''Quercus ilex'') and Norway spruce (''Picea abies''). It has a strained ring system with a cyclopentane ring fused to a cyclopropane ring. Sabinene is one of the chemical compounds that contributes to the spiciness of black pepper and is a major constituent of carrot seed oil. It also occurs in tea tree oil at a low concentration. It is also present in the essential oil obtained from nutmeg, '' Laurus nobilis'', and '' Clausena anisata''. Biosynthesis Sabinene, a bicyclic monoterpene, is present in the (+) and (-) enantiomer In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical ant ...s. It is biosynthesized from th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sabinene BIosynthesis
Sabinene is a natural bicyclic monoterpene with the molecular formula C10H16. It is isolated from the essential oils of a variety of plants including Marjoram, holm oak (''Quercus ilex'') and Norway spruce (''Picea abies''). It has a strained ring system with a cyclopentane ring fused to a cyclopropane ring. Sabinene is one of the chemical compounds that contributes to the spiciness of black pepper and is a major constituent of carrot seed oil. It also occurs in tea tree oil at a low concentration. It is also present in the essential oil obtained from nutmeg, ''Laurus nobilis'', and ''Clausena anisata''. Biosynthesis Sabinene, a bicyclic monoterpene, is present in the (+) and (-) enantiomers. It is biosynthesized from the common terpenoid precursor, geranyl pyrophosphate (GPP) that undergoes polycyclization catalyzed by sabinene synthase (SabS). GPP is formed from the terpenoid synthesis pathway with the starter units, isopentenyl pyrophosphate (IPP) and dimethylallyl pyroph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoterpenes
Monoterpenes are a class of terpenes that consist of two isoprene units and have the molecular formula C10H16. Monoterpenes may be linear (acyclic) or contain rings (monocyclic and bicyclic). Modified terpenes, such as those containing oxygen functionality or missing a methyl group, are called monoterpenoids. Monoterpenes and monoterpenoids are diverse. They have relevance to the pharmaceutical, cosmetic, agricultural, and food industries. Biosynthesis Monoterpenes are derived biosynthetically from units of isopentenyl pyrophosphate, which is formed from acetyl-CoA via the intermediacy of mevalonic acid in the HMG-CoA reductase pathway. An alternative, unrelated biosynthesis pathway of IPP is known in some bacterial groups and the plastids of plants, the so-called MEP-(2-methyl-D-erythritol-4-phosphate) pathway, which is initiated from C5 sugars. In both pathways, IPP is isomerized to DMAPP by the enzyme isopentenyl pyrophosphate isomerase. Geranyl pyrophosphate is the precursor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clausena Anisata
:''Should not be confused with syzygium anisatum, a tree native to eastern Australian rainforests, used as a culinary herb.'' ''Clausena anisata'' ( Willd.) Hook.f. ex Benth. is a deciduous shrub or small tree, belonging to the Rutaceae or Citrus family, and widespread in the Afrotropical realm or Sub-Saharan Africa, but absent from the drier regions. It is also found in tropical and South-East Asia, growing in India and Sri Lanka and extending as far as Queensland in north-eastern Australia and some Pacific islands. It is cultivated in Malaysia and Indonesia. As with other plants useful to mankind its large range of medicinal properties has led to a global distribution and its growth wherever the climate is suitable. It grows in higher-rainfall regions in savanna, thickets, riverine forest, disturbed areas and secondary forest, up to an altitude of 3000 m. The leaves, which are foetid when bruised, give rise to the common name 'Horsewood' or the more descriptive Afrikaans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoterpene
Monoterpenes are a class of terpenes that consist of two isoprene units and have the molecular formula C10H16. Monoterpenes may be linear (acyclic) or contain rings (monocyclic and bicyclic). Modified terpenes, such as those containing oxygen functionality or missing a methyl group, are called monoterpenoids. Monoterpenes and monoterpenoids are diverse. They have relevance to the pharmaceutical, cosmetic, agricultural, and food industries. Biosynthesis Monoterpenes are derived biosynthetically from units of isopentenyl pyrophosphate, which is formed from acetyl-CoA via the intermediacy of mevalonic acid in the HMG-CoA reductase pathway. An alternative, unrelated biosynthesis pathway of IPP is known in some bacterial groups and the plastids of plants, the so-called MEP-(2-methyl-D-erythritol-4-phosphate) pathway, which is initiated from C5 sugars. In both pathways, IPP is isomerized to DMAPP by the enzyme isopentenyl pyrophosphate isomerase. Geranyl pyrophosphate is the precurs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thujene
Thujene (or α-thujene) is a natural organic compound classified as a monoterpene. It is found in the essential oils of a variety of plants, and contributes pungency to the flavor of some herbs such as Summer savory.''PDR for Herbal Medicines'', Third Edition, Joerg Gruenwald (Editor), page 802. The term ''thujene'' usually refers to α-thujene. A less common chemically related double-bond isomer In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers. Iso ... is known as β-thujene (or 2-thujene). Another double-bond isomer is known as sabinene. {, class="toccolours" border="1" style="margin: 0 0 1em 1em; border-collapse: collapse;" , colspan="3" align="center" , Chemical structure comparison , - , , , , - , align="center", α-Thujene , align="center", β-Thujene , align="cent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beilstein Database
The Beilstein database is the largest database in the field of organic chemistry, in which compounds are uniquely identified by their Beilstein Registry Number. The database covers the scientific literature from 1771 to the present and contains experimentally validated information on millions of chemical reactions and substances from original scientific publications. The electronic database was created from ''Handbuch der Organischen Chemie'' (''Beilstein's Handbook of Organic Chemistry''), founded by Friedrich Konrad Beilstein in 1881, but has appeared online under a number of different names, including Crossfire Beilstein. Since 2009, the content has been maintained and distributed by Elsevier Information Systems in Frankfurt under the product name " Reaxys". The database contains information on reactions, substances, structures and properties. Up to 350 fields containing chemical and physical data (such as melting point, refractive index etc.) are available for each substance. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopentenyl Pyrophosphate
Isopentenyl pyrophosphate (IPP, isopentenyl diphosphate, or IDP) is an isoprenoid precursor. IPP is an intermediate in the classical, HMG-CoA reductase pathway (commonly called the mevalonate pathway) and in the ''non-mevalonate'' MEP pathway of isoprenoid precursor biosynthesis. Isoprenoid precursors such as IPP, and its isomer DMAPP, are used by organisms in the biosynthesis of terpenes and terpenoids. Biosynthesis IPP is formed from acetyl-CoA via the mevalonate pathway (the "upstream" part), and then is isomerized to dimethylallyl pyrophosphate by the enzyme isopentenyl pyrophosphate isomerase. IPP can be synthesised via an alternative non-mevalonate pathway of isoprenoid precursor biosynthesis, the MEP pathway, where it is formed from (''E'')-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP) by the enzyme HMB-PP reductase (LytB, IspH). The MEP pathway is present in many bacteria, apicomplexan protozoa such as malaria parasites, and in the plastids of higher plan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropanes
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane itself is mainly of theoretical interest but many of its derivatives are of commercial or biological significance. History Cyclopropane was discovered in 1881 by August Freund, who also proposed the correct structure for the substance in his first paper. Freund treated 1,3-dibromopropane with sodium, causing an intramolecular Wurtz reaction leading directly to cyclopropane. The yield of the reaction was improved by Gustavson in 1887 with the use of zinc instead of sodium. Cyclopropane had no commercial application until Henderson and Lucas discovered its anaesthetic properties in 1929; industrial production had begun by 1936. In modern anaesthetic practice, it has been superseded by other agents. Anaesthesia Cyclopropane was introduced into clin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene Derivatives
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocarbons
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or exemplified by the odors of gasoline and lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases (such as methane and propane), liquids (such as hexane and benzene), low melting solids (such as paraffin wax and naphthalene) or polymers (such as polyethylene and polystyrene). In the fossil fuel industries, ''hydrocarbon'' refers to the naturally occurring petroleum, natural gas and coal, and to their hydrocarbon derivatives and purified forms. Combustion of hydrocarbons is the main source of the world's energy. Petroleum is the dominant raw-material source for organic commodity chemicals such as solvents and polymers. Most anthropogenic (human-generated) emissions of greenhouse gases ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MEP Pathway
The non-mevalonate pathway—also appearing as the mevalonate-independent pathway and the 2-''C''-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate (MEP/DOXP) pathway—is an alternative metabolic pathway for the biosynthesis of the isoprenoid precursors isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). The currently preferred name for this pathway is the MEP pathway, since MEP is the first committed metabolite on the route to IPP. Isoprenoid precursor biosynthesis The classical mevalonate pathway (MVA pathway or HMG-CoA reductase pathway) is a metabolic pathway for the biosynthesis of isoprenoid precursors: IPP and DMAPP. The MVA pathway is present in most eukaryotes and some bacteria. IPP and DMAPP serve as the basis for the biosynthesis of isoprenoid (terpenoid) molecules used in processes as diverse as protein prenylation, cell membrane maintenance, the synthesis of hormones, protein anchoring and ''N''-glycosylation in all three domains ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
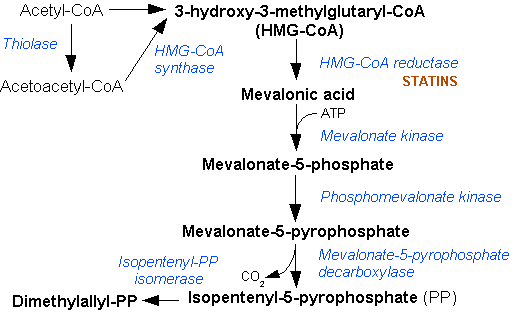
Mevalonate Pathway
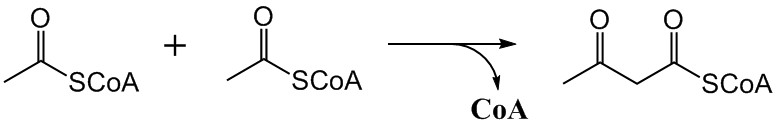
The mevalonate pathway, also known as the isoprenoid pathway or HMG-CoA reductase pathway is an essential metabolic pathway present in eukaryotes, archaea, and some bacteria. The pathway produces two five-carbon building blocks called isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), which are used to make isoprenoids, a diverse class of over 30,000 biomolecules such as cholesterol, vitamin K, coenzyme Q10, and all steroid hormones. The mevalonate pathway begins with acetyl-CoA and ends with the production of IPP and DMAPP. It is best known as the target of statins, a class of cholesterol lowering drugs. Statins inhibit HMG-CoA reductase within the mevalonate pathway. Upper mevalonate pathway The mevalonate pathway of eukaryotes, archaea, and eubacteria all begin the same way. The sole carbon feed stock of the pathway is acetyl-CoA. The first step condenses two acetyl-CoA molecules to yield acetoacetyl-CoA. This is followed by a second condensation to fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |