|
Thujene
Thujene (or α-thujene) is a natural organic compound classified as a monoterpene. It is found in the essential oils of a variety of plants, and contributes pungency to the flavor of some herbs such as Summer savory.''PDR for Herbal Medicines'', Third Edition, Joerg Gruenwald (Editor), page 802. The term ''thujene'' usually refers to α-thujene. A less common chemically related double-bond isomer In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers. Iso ... is known as β-thujene (or 2-thujene). Another double-bond isomer is known as sabinene. {, class="toccolours" border="1" style="margin: 0 0 1em 1em; border-collapse: collapse;" , colspan="3" align="center" , Chemical structure comparison , - , , , , - , align="center", α-Thujene , align="center", β-Thujene , align="cent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoterpene
Monoterpenes are a class of terpenes that consist of two isoprene units and have the molecular formula C10H16. Monoterpenes may be linear (acyclic) or contain rings (monocyclic and bicyclic). Modified terpenes, such as those containing oxygen functionality or missing a methyl group, are called monoterpenoids. Monoterpenes and monoterpenoids are diverse. They have relevance to the pharmaceutical, cosmetic, agricultural, and food industries. Biosynthesis Monoterpenes are derived biosynthetically from units of isopentenyl pyrophosphate, which is formed from acetyl-CoA via the intermediacy of mevalonic acid in the HMG-CoA reductase pathway. An alternative, unrelated biosynthesis pathway of IPP is known in some bacterial groups and the plastids of plants, the so-called MEP-(2-methyl-D-erythritol-4-phosphate) pathway, which is initiated from C5 sugars. In both pathways, IPP is isomerized to DMAPP by the enzyme isopentenyl pyrophosphate isomerase. Geranyl pyrophosphate is the precurs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bicyclic Compounds
In chemistry, a bicyclic molecule () is a molecule that features two joined rings. Bicyclic structures occur widely, for example in many biologically important molecules like α-thujene and camphor. A bicyclic compound can be carbocyclic (all of the ring atoms are carbons), or heterocyclic (the rings' atoms consist of at least two elements), like DABCO. Moreover, the two rings can both be aliphatic (''e.g.'' decalin and norbornane), or can be aromatic (''e.g.'' naphthalene), or a combination of aliphatic and aromatic (''e.g.'' tetralin). Three modes of ring junction are possible for a bicyclic compound: * In spirocyclic compounds, the two rings share only one single atom, the spiro atom, which is usually a quaternary carbon. An example of a spirocyclic compound is the photochromic switch spiropyran. * In fused/condensed bicyclic compounds, two rings share two adjacent atoms. In other words, the rings share one covalent bond, ''i.e.'' the so-called bridgehead atoms are direc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoterpenes
Monoterpenes are a class of terpenes that consist of two isoprene units and have the molecular formula C10H16. Monoterpenes may be linear (acyclic) or contain rings (monocyclic and bicyclic). Modified terpenes, such as those containing oxygen functionality or missing a methyl group, are called monoterpenoids. Monoterpenes and monoterpenoids are diverse. They have relevance to the pharmaceutical, cosmetic, agricultural, and food industries. Biosynthesis Monoterpenes are derived biosynthetically from units of isopentenyl pyrophosphate, which is formed from acetyl-CoA via the intermediacy of mevalonic acid in the HMG-CoA reductase pathway. An alternative, unrelated biosynthesis pathway of IPP is known in some bacterial groups and the plastids of plants, the so-called MEP-(2-methyl-D-erythritol-4-phosphate) pathway, which is initiated from C5 sugars. In both pathways, IPP is isomerized to DMAPP by the enzyme isopentenyl pyrophosphate isomerase. Geranyl pyrophosphate is the precursor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon- hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Livin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Umbellulone
Umbellulone is a headache-inducing monoterpene ketone found in the leaves of the tree '' Umbellularia californica'', sometimes known as the "headache tree". It is hypothesized to cause headaches by influencing the trigeminovascular system via TRPA1. History '' Umbellularia californica'' is a tree native to the coastal forest of California. Botanist Archibald Menzies was the first to collect the oil at the end of the 18th century. In 1826 this tree was classified as a laurel, ''Laurus regia'', by botanist David Douglas. In 1833 the tree received another classification by Hooker and Arnott, ''Tetranthera californica''. Shortly after that the present name was given by Nuttal, ''Umbellularia californica''. In 1875 Heaney obtained a colorless liquid (oreodaphenol) via fractionation under reduced pressure. This oil of the California laurel possess a pungent odor. Stillman (1880) did a fractionation at 215-216 °C. He discovered that inhalation of its fumes can lead to a pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thujone
Thujone () is a ketone and a monoterpene that occurs predominantly in two diastereomeric ( epimeric) forms: (−)-α-thujone and (+)-β-thujone. Though it is best known as a chemical compound in the spirit absinthe, it is unlikely to be responsible for absinthe's alleged stimulant and psychoactive effects due to the small quantities present. Thujone acts on the neurotransmitter gamma-aminobutyric acid (GABA) as an antagonist (opposite to the effects of alcohol). As a competitive antagonist of GABA, thujone alone is considered to be convulsant, though by interfering with the inhibitory transmitter GABA, it may convey stimulating, mood-elevating effects at low doses. It is also used in perfumery as a component of several essential oils. In addition to the naturally occurring (−)-α-thujone and (+)-β-thujone, two other forms are possible: (+)-α-thujone and (−)-β-thujone. In 2016, they were found in nature as well, in '' Salvia officinalis''. File:(-)-alpha-Thujon.svg, ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
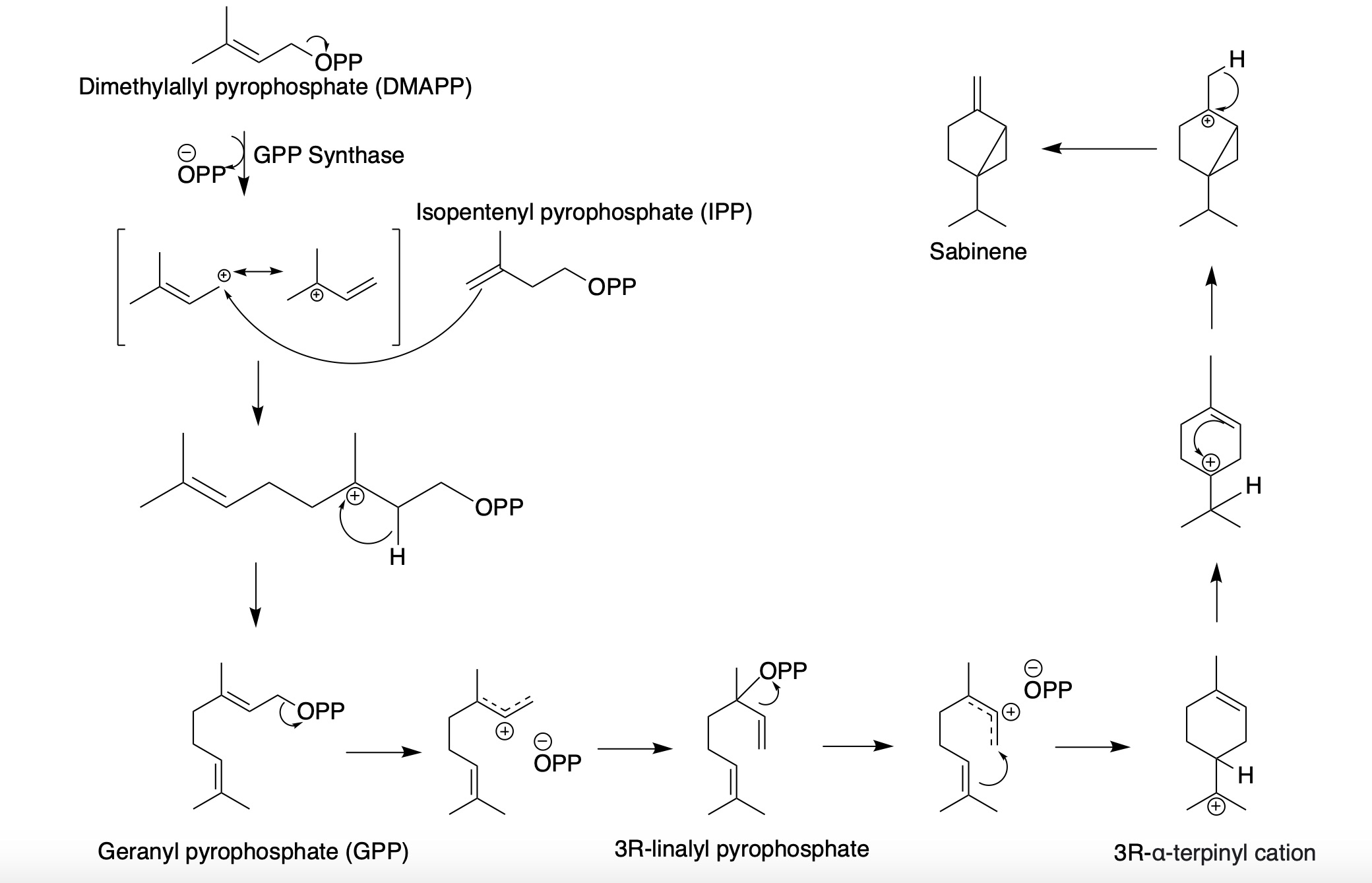
Sabinene
Sabinene is a natural bicyclic monoterpene with the molecular formula C10H16. It is isolated from the essential oils of a variety of plants including Marjoram, holm oak (''Quercus ilex'') and Norway spruce (''Picea abies''). It has a strained ring system with a cyclopentane ring fused to a cyclopropane ring. Sabinene is one of the chemical compounds that contributes to the spiciness of black pepper and is a major constituent of carrot seed oil. It also occurs in tea tree oil at a low concentration. It is also present in the essential oil obtained from nutmeg, '' Laurus nobilis'', and '' Clausena anisata''. Biosynthesis Sabinene, a bicyclic monoterpene, is present in the (+) and (-) enantiomer In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical ant ...s. It is biosynthesized from th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sabinene
Sabinene is a natural bicyclic monoterpene with the molecular formula C10H16. It is isolated from the essential oils of a variety of plants including Marjoram, holm oak (''Quercus ilex'') and Norway spruce (''Picea abies''). It has a strained ring system with a cyclopentane ring fused to a cyclopropane ring. Sabinene is one of the chemical compounds that contributes to the spiciness of black pepper and is a major constituent of carrot seed oil. It also occurs in tea tree oil at a low concentration. It is also present in the essential oil obtained from nutmeg, '' Laurus nobilis'', and '' Clausena anisata''. Biosynthesis Sabinene, a bicyclic monoterpene, is present in the (+) and (-) enantiomer In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical ant ...s. It is biosynthesized from th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers. Isomers do not necessarily share similar chemical or physical properties. Two main forms of isomerism are structural or constitutional isomerism, in which '' bonds'' between the atoms differ; and stereoisomerism or spatial isomerism, in which the bonds are the same but the ''relative positions'' of the atoms differ. Isomeric relationships form a hierarchy. Two chemicals might be the same constitutional isomer, but upon deeper analysis be stereoisomers of each other. Two molecules that are the same stereoisomer as each other might be in different conformational forms or be different isotopologues. The depth of analysis depends on the field of study or the chemical and physical properties of interest. The English word "isomer" () is a bac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |