|
Monoterpene
Monoterpenes are a class of terpenes that consist of two isoprene units and have the molecular formula C10H16. Monoterpenes may be linear (acyclic) or contain rings (monocyclic and bicyclic). Modified terpenes, such as those containing oxygen functionality or missing a methyl group, are called monoterpenoids. Monoterpenes and monoterpenoids are diverse. They have relevance to the pharmaceutical, cosmetic, agricultural, and food industries. Biosynthesis Monoterpenes are derived biosynthetically from units of isopentenyl pyrophosphate, which is formed from acetyl-CoA via the intermediacy of mevalonic acid in the HMG-CoA reductase pathway. An alternative, unrelated biosynthesis pathway of IPP is known in some bacterial groups and the plastids of plants, the so-called MEP-(2-methyl-D-erythritol-4-phosphate) pathway, which is initiated from C5 sugars. In both pathways, IPP is isomerized to DMAPP by the enzyme isopentenyl pyrophosphate isomerase. Geranyl pyrophosphate is the precurso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terpene
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n > 1. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes are further classified by the number of carbons: monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), as examples. The terpene alpha-pinene, is a major component of the common solvent, turpentine. History and terminology The term ''terpene'' was coined in 1866 by the German chemist August Kekulé to denote all hydrocarbons having the empirical formula C10H16, of which camphene was one. Previously, many hydrocarbons having the empirical formula C10H16 had been called "camphene", but many other hydrocarbons of the same composition had had different names. Kekulé coined the term "terpene" in order to reduce the confusion. The name "terpene" is a shortened form of "terpentine", an obsolete spelling of "turpentine". Although sometimes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halomon
Halomon is a polyhalogenated monoterpene first isolated from the marine red algae '' Portieria hornemannii''. Halomon has attracted research interest because of its promising profile of selective cytotoxicity that suggests its potential use as an antitumor agent. Halomon is in a class of chemical compounds known as halocarbons, which are often potent alkylating agents which may be toxic to individual cells or to living organisms. The red algae that naturally produce halomon and other related compounds probably do so as a poisonous defense against fish or other marine life that may see it as a potential source of food. Halomon, however, is a selective toxin; studies at the National Cancer Institute have indicated that it is more toxic to certain types of tumor cells than to other cells. The algae that produces halomon is difficult to locate, identify, and collect and the concentration of halomon in the organism is extremely low. Therefore, obtaining a sufficient amount of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
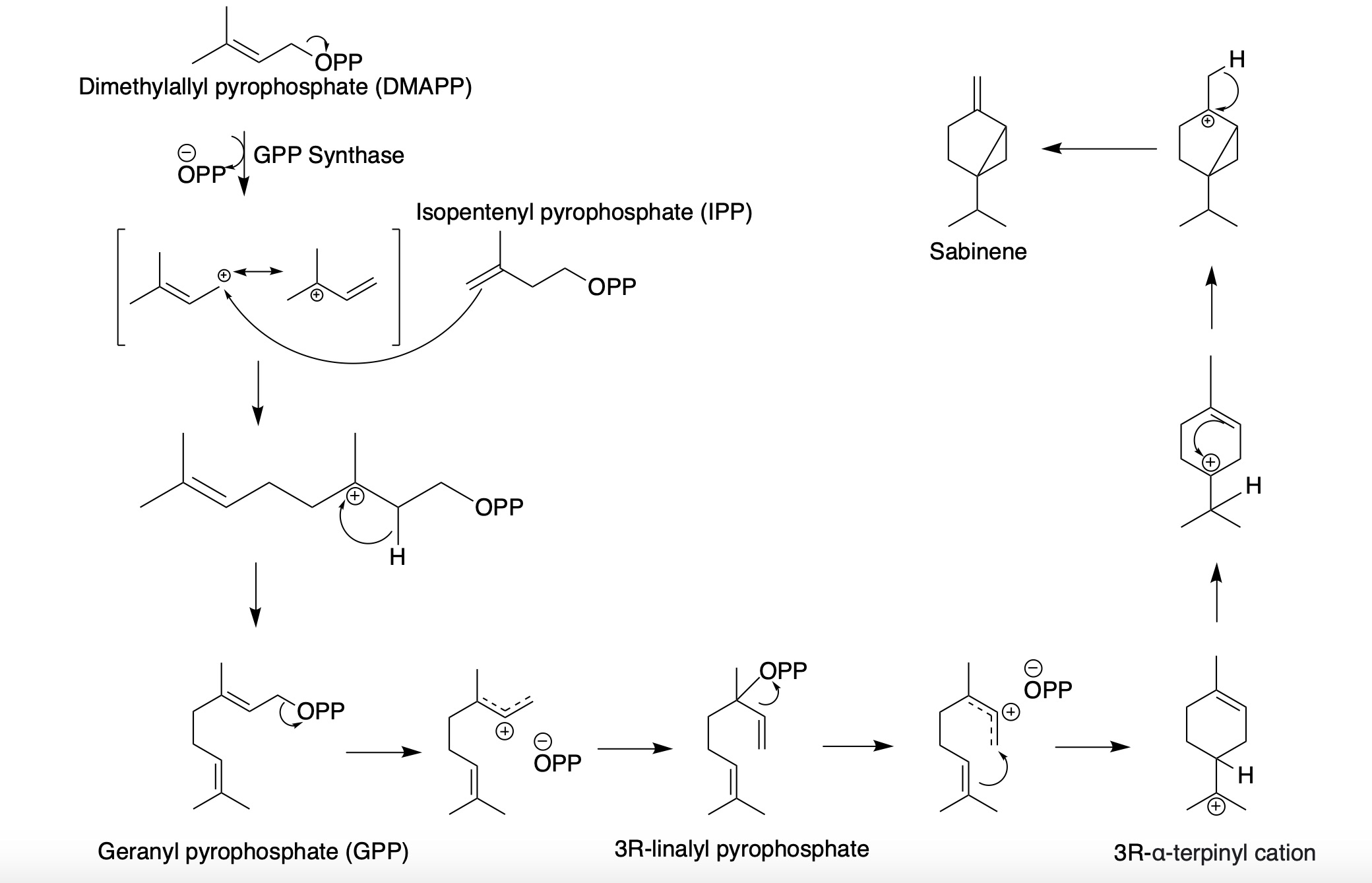
Sabinene
Sabinene is a natural bicyclic monoterpene with the molecular formula C10H16. It is isolated from the essential oils of a variety of plants including Marjoram, holm oak (''Quercus ilex'') and Norway spruce (''Picea abies''). It has a strained ring system with a cyclopentane ring fused to a cyclopropane ring. Sabinene is one of the chemical compounds that contributes to the spiciness of black pepper and is a major constituent of carrot seed oil. It also occurs in tea tree oil at a low concentration. It is also present in the essential oil obtained from nutmeg, ''Laurus nobilis'', and ''Clausena anisata''. Biosynthesis Sabinene, a bicyclic monoterpene, is present in the (+) and (-) enantiomers. It is biosynthesized from the common terpenoid precursor, geranyl pyrophosphate (GPP) that undergoes polycyclization catalyzed by sabinene synthase (SabS). GPP is formed from the terpenoid synthesis pathway with the starter units, isopentenyl pyrophosphate (IPP) and dimethylallyl py ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myrcene
Myrcene, or β-myrcene, is a monoterpene. A colorless oil, it occurs widely in essential oils. It is produced mainly semi-synthetically from '' Myrcia'', from which it gets its name. It is an intermediate in the production of several fragrances. α-Myrcene is the name for the isomer 2-methyl-6-methylene-1,7-octadiene, which has not been found in nature. Production Myrcene is often produced commercially by the pyrolysis (400 °C) of β-pinene, which is obtained from turpentine. It is rarely obtained directly from plants. Plants biosynthesize myrcene via geranyl pyrophosphate (GPP), which isomerizes into linalyl pyrophosphate. The release of the pyrophosphate (OPP) and a proton completes the conversion. Occurrence It could in principle be extracted from any number of plants, such as verbena or wild thyme, the leaves of which contain up to 40% by weight of myrcene. Many other plants contain myrcene, sometimes in substantial amounts. Some of these include cannabis, hops, ''Hou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carvone
Carvone is a member of a family of chemicals called terpenoids. Carvone is found naturally in many essential oils, but is most abundant in the oils from seeds of caraway (''Carum carvi''), spearmint (''Mentha spicata''), and dill. Uses Both carvones are used in the food and flavor industry. ''R''-(−)-Carvone is also used for air freshening products and, like many essential oils, oils containing carvones are used in aromatherapy and alternative medicine. ''S''-(+)-Carvone has shown a suppressant effect against high-fat diet induced weight gain in mice. Food applications As the compound most responsible for the flavor of caraway, dill and spearmint, carvone has been used for millennia in food. Wrigley's Spearmint Gum and spearmint flavored Life Savers are major users of natural spearmint oil from ''Mentha spicata''. Caraway seed is extracted with alcohol to make the European drink Kümmel. Agriculture ''S''-(+)-Carvone is also used to prevent premature sprouting of potatoes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Limonene
Limonene is a colorless liquid aliphatic hydrocarbon classified as a cyclic monoterpene, and is the major component in the oil of citrus fruit peels. The -isomer, occurring more commonly in nature as the fragrance of oranges, is a flavoring agent in food manufacturing. It is also used in chemical synthesis as a precursor to carvone and as a renewables-based solvent in cleaning products. The less common -isomer has a piny, turpentine-like odor, and is found in the edible parts of such plants as caraway, dill, and bergamot orange plants. Limonene takes its name from Italian ''limone'' ("lemon"). Limonene is a chiral molecule, and biological sources produce one enantiomer: the principal industrial source, citrus fruit, contains -limonene ((+)-limonene), which is the (''R'')-enantiomer. Racemic limonene is known as dipentene. -Limonene is obtained commercially from citrus fruits through two primary methods: centrifugal separation or steam distillation. Chemical reactions Limon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citral
Citral is an acyclic monoterpene aldehyde, and being a monoterpene, it is made of two isoprene units. Citral is a collective term which covers two geometric isomers that have their own separate names; the ''E''-isomer is named geranial (''trans''-citral) or citral A. The ''Z''-isomer is named neral (''cis''-citral) or citral B. These stereoisomers occur as a mixture, not necessarily racemic; e.g. in essential oil of Australian ginger, the neral to geranial ratio is 0.61. Occurrence Citral is present in the oils of several plants, including lemon myrtle (90–98%), '' Litsea citrata'' (90%), ''Litsea cubeba'' (70–85%), lemongrass (65–85%), lemon tea-tree (70–80%), ''Ocimum gratissimum'' (66.5%), '' Lindera citriodora'' (about 65%), '' Calypranthes parriculata'' (about 62%), petitgrain (36%), lemon verbena (30–35%), lemon ironbark (26%), lemon balm (11%), lime (6–9%), lemon (2–5%), and orange. Further, in the lipid fraction (essential oil) of Australian ginger (51- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myrcene
Myrcene, or β-myrcene, is a monoterpene. A colorless oil, it occurs widely in essential oils. It is produced mainly semi-synthetically from '' Myrcia'', from which it gets its name. It is an intermediate in the production of several fragrances. α-Myrcene is the name for the isomer 2-methyl-6-methylene-1,7-octadiene, which has not been found in nature. Production Myrcene is often produced commercially by the pyrolysis (400 °C) of β-pinene, which is obtained from turpentine. It is rarely obtained directly from plants. Plants biosynthesize myrcene via geranyl pyrophosphate (GPP), which isomerizes into linalyl pyrophosphate. The release of the pyrophosphate (OPP) and a proton completes the conversion. Occurrence It could in principle be extracted from any number of plants, such as verbena or wild thyme, the leaves of which contain up to 40% by weight of myrcene. Many other plants contain myrcene, sometimes in substantial amounts. Some of these include cannabis, hops, ''Hou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ocimene
Ocimenes are a group of isomeric hydrocarbons. The ocimenes are monoterpenes found within a variety of plants and fruits. α-Ocimene and the two β-ocimenes differ in the position of the isolated double bond: it is terminal in the alpha isomer. α-Ocimene is ''cis-''3,7-dimethyl-1,3,7-octatriene. β-Ocimene is ''trans-''3,7-dimethyl-1,3,6-octatriene. β-Ocimene exists in two stereoisomeric forms, ''cis'' and ''trans,'' with respect to the central double bond. The ocimenes are often found naturally as mixtures of the various forms. The mixture, as well as the pure compounds, are oils with a pleasant odor. They are used in perfumery for their sweet herbal scent, and are believed to act as plant defense and have anti-fungal properties. Like the related acyclic terpene myrcene, ocimenes are unstable in air.Karl-Georg Fahlbusch, Franz-Josef Hammerschmidt, Johannes Panten, Wilhelm Pickenhagen, Dietmar Schatkowski, Kurt Bauer, Dorothea Garbe, Horst Surburg "Flavors and Fragrances ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thujene
Thujene (or α-thujene) is a natural organic compound classified as a monoterpene. It is found in the essential oils of a variety of plants, and contributes pungency to the flavor of some herbs such as Summer savory.''PDR for Herbal Medicines'', Third Edition, Joerg Gruenwald (Editor), page 802. The term ''thujene'' usually refers to α-thujene. A less common chemically related double-bond isomer is known as β-thujene (or 2-thujene). Another double-bond isomer is known as sabinene. {, class="toccolours" border="1" style="margin: 0 0 1em 1em; border-collapse: collapse;" , colspan="3" align="center" , Chemical structure comparison , - , , , , - , align="center", α-Thujene , align="center", β-Thujene , align="center", Sabinene See also * Thujone * Umbellulone Umbellulone is a headache-inducing monoterpene ketone found in the leaves of the tree ''Umbellularia californica'', sometimes known as the "headache tree". It is hypothesized to cause headaches by influenci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Camphene
Camphene is a bicyclic organic compound. It is one of the most pervasive monoterpenes. As for other terpenes, it is insoluble in water, flammable, colorless, and has a pungent smell. It is a minor constituent of many essential oils such as turpentine, cypress oil, camphor oil, citronella oil, neroli, ginger oil, valerian, and mango. It is produced industrially by isomerization of the more common alpha-pinene using a solid acid catalyst such as titanium dioxide. Camphene is used in the preparation of fragrances and as a food additive for flavoring. These include isobornyl acetate. Biosynthesis Camphene is biosynthesized from linalyl pyrophosphate via a sequence of carbocation A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountere ...ic intermediates. References {{Authority con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carene
3-Carene is a bicyclic monoterpene consisting of fused cyclohexene and cyclopropane rings. It occurs as a constituent of turpentine, with a content as high as 42% depending on the source. Carene has a sweet and pungent odor, best described as a combination of fir needles, musky earth, and damp woodlands. A colorless liquid, it is not soluble in water, but miscible with fats and oils. It is chiral, occurring naturally both as the racemate and enantio-enriched forms. Reactions and uses Treatment with peracetic acid gives 3,4-caranediol. Pyrolysis over ferric oxide induces rearrangement, giving ''p''-cymene. Carene is used in the perfume industry and as a chemical intermediate. Because carene can be found in cannabis naturally, it can also be found in cannabis distillates. Greater concentrations of carene in a distillate give it an earthier taste and smell. 3-Carene is also present in mango, giving the fruit a characteristic pine A pine is any conifer tree or shrub in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |