|
Pericyclic
In organic chemistry, a pericyclic reaction is the type of organic reaction wherein the transition state of the molecule has a cyclic geometry, the reaction progresses in a concerted fashion, and the bond orbitals involved in the reaction overlap in a continuous cycle at the transition state. Pericyclic reactions stand in contrast to ''linear reactions'', encompassing most organic transformations and proceeding through an acyclic transition state, on the one hand and '' coarctate reactions'', which proceed through a doubly cyclic, concerted transition state on the other hand. Pericyclic reactions are usually rearrangement or addition reactions. The major classes of pericyclic reactions are given in the table below (the three most important classes are shown in bold). Ene reactions and cheletropic reactions are often classed as group transfer reactions and cycloadditions/cycloeliminations, respectively, while dyotropic reactions and group transfer reactions (if ene reactions are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Woodward–Hoffmann Rules
The Woodward–Hoffmann rules (or the pericyclic selection rules), devised by Robert Burns Woodward and Roald Hoffmann, are a set of rules used to rationalize or predict certain aspects of the stereochemistry and activation energy of pericyclic reactions, an important class of reactions in organic chemistry. The rules are best understood in terms of the concept of ''the conservation of orbital symmetry'' using ''orbital correlation diagrams'' (see Section 3 below). The Woodward–Hoffmann rules are a consequence of the changes in electronic structure that occur during a pericyclic reaction and are predicated on the phasing of the interacting molecular orbitals. They are applicable to all classes of pericyclic reactions (and their microscopic reverse 'retro' processes), including (1) electrocyclic reaction, electrocyclizations, (2) cycloadditions, (3) sigmatropic reactions, (4) group transfer reactions, (5) ene reactions, (6) cheletropic reactions, and (7) dyotropic reactions. Due to t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pericyclic Arrow Pushing
In organic chemistry, a pericyclic reaction is the type of organic reaction wherein the transition state of the molecule has a cyclic geometry, the reaction progresses in a concerted fashion, and the bond orbitals involved in the reaction overlap in a continuous cycle at the transition state. Pericyclic reactions stand in contrast to ''linear reactions'', encompassing most organic transformations and proceeding through an acyclic transition state, on the one hand and '' coarctate reactions'', which proceed through a doubly cyclic, concerted transition state on the other hand. Pericyclic reactions are usually rearrangement or addition reactions. The major classes of pericyclic reactions are given in the table below (the three most important classes are shown in bold). Ene reactions and cheletropic reactions are often classed as group transfer reactions and cycloadditions/cycloeliminations, respectively, while dyotropic reactions and group transfer reactions (if ene reactions are e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, Mechanistic Organic Photochemistry, photochemical reactions and organic redox reaction, redox reactions. In organic synthesis, organic reactions are used in the construction of new organic molecules. The production of many man-made chemicals such as drugs, plastics, food additives, fabrics depend on organic reactions. The oldest organic reactions are combustion of organic fuels and saponification of fats to make soap. Modern organic chemistry starts with the Wöhler synthesis in 1828. In the history of the Nobel Prize in Chemistry awards have been given for the invention of specific organic reactions such as the Grignard reaction in 1912, the Diels-Alder reaction in 1950, the Wittig reaction in 1979 and olefin metathesis in 2005. Classifications Organic c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coarctate Reaction
In the classification of organic reactions by transition state topology, a coarctate reaction (from L. ''coarctare'' "to constrict") is a third, comparatively uncommon topology, after linear topology and pericyclic topology (itself subdivided into Hückel and Möbius topologies). Transition state topologies Reactions of linear topology are the most common, and consist of all transformations whose transition states are acyclic, including addition, elimination, substitution, and (some types of) fragmentation reactions. In contrast, in pericyclic reactions, the atoms under chemical change form a closed cycle, and include reactions like the Diels-Alder reaction and Cope rearrangement, among many others. In contrast to these types of reactions, a coarctate reaction is characterized by a doubly cyclic transition state, in which at least one atom undergoes the simultaneous making and breaking of two bonds. Thus, the topology of the transition state of a coarctate reaction is a constr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Möbius Aromaticity
In organic chemistry, Möbius aromaticity is a special type of aromaticity believed to exist in a number of organic molecules. In terms of molecular orbital theory these compounds have in common a monocyclic array of molecular orbitals in which there is an odd number of out-of-phase overlaps, the opposite pattern compared to the aromatic character to Hückel's rule, Hückel systems. The nodal plane of the orbitals, viewed as a ribbon, is a Möbius strip, rather than a cylinder, hence the name. The pattern of orbital energies is given by a rotated Möbius–Hückel concept, Frost circle (with the edge of the polygon on the bottom instead of a vertex), so systems with 4''n'' electrons are aromatic, while those with 4''n'' + 2 electrons are anti-aromatic/non-aromatic. Due to incrementally twisted nature of the orbitals of a Möbius aromatic system, stable Möbius aromatic molecules need to contain at least 8 electrons, although 4 electron Möbius aromatic transition states are well k ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the Multiplicity (chemistry)#Molecules, bond multiplicity". The resulting reaction is a cyclization reaction. Many but not all cycloadditions are Concerted reaction, concerted and thus pericyclic. Nonconcerted cycloadditions are not pericyclic. As a class of addition reaction, cycloadditions permit carbon–carbon bond formation without the use of a nucleophile or electrophile. Cycloadditions can be described using two systems of notation. An older but still common notation is based on the size of linear arrangements of atoms in the reactants. It uses parentheses: where the variables are the numbers of linear atoms in each reactant. The product is a cycle of size . In this system, the standard Diels-Alder reaction is a (4 + 2)-cyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rearrangement Reaction
In organic chemistry, a rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule, hence these reactions are usually intramolecular. In the example below, the substituent R moves from carbon atom 1 to carbon atom 2: :\underset\ce\ce\underset\ce\ce Intermolecular rearrangements also take place. A rearrangement is not well represented by simple and discrete electron transfers (represented by curved arrows in organic chemistry texts). The actual mechanism of alkyl groups moving, as in Wagner-Meerwein rearrangement, probably involves transfer of the moving alkyl group fluidly along a bond, not ionic bond-breaking and forming. In pericyclic reactions, explanation by orbital interactions give a better picture than simple discrete electron transfers. It is, nevertheless, possible to draw the curv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cheletropic Reaction
In organic chemistry, cheletropic reactions, also known as chelotropic reactions, IUPAC GoldBook are a type of pericyclic reaction (a chemical reaction that involves a transition state with a Ring (chemistry), cyclic array of atoms and an associated cyclic array of interacting Atomic orbital, orbitals).Eric V. Anslyn and Dennis A. Dougherty ''Modern Physical Organic Chemistry'' University Science Books, 2006. Specifically, cheletropic reactions are a subclass of cycloadditions. The key distinguishing feature of cheletropic reactions is that on one of the reagents, both new bonds are being made to the same atom.Ian Fleming. ''Frontier Orbitals and Organic Chemistry Reactions.'' Wiley, 1976. Theoretical analysis In the pericyclic transition state, a small molecu ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
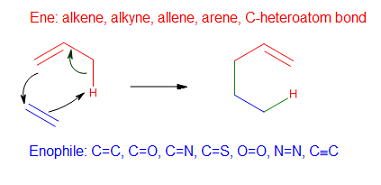
Group Transfer Reaction
In organic chemistry, a group transfer reaction is a pericyclic process where one or more groups of atoms is transferred from one molecule to another. They can sometimes be difficult to identify when separate reactant molecules combine into a single product molecule (like in the ene reaction). Unlike other pericyclic reaction classes, group transfer reactions do not have a specific conversion of pi bonds into sigma bonds or vice versa, and tend to be less frequently encountered. Like all pericyclic reactions, they must obey the Woodward–Hoffmann rules. The best known group transfer reaction is the ene reaction In organic chemistry, the ene reaction (also known as the Alder-ene reaction by its discoverer Kurt Alder in 1943) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile ... in which an allylic hydrogen is transferred to an alkene. References Rearrangement reactions Pericyclic reactions [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Claisen Rearrangement
The Claisen rearrangement is a powerful carbon–carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen. The heating of an allyl vinyl ether will initiate a ,3sigmatropic rearrangement to give a γ,δ-unsaturated carbonyl, driven by exergonically favored carbonyl CO bond formation (ΔΔHf = -327kcalmol−1). Mechanism The Claisen rearrangement is an exothermic, concerted (bond cleavage and recombination) pericyclic reaction. Woodward–Hoffmann rules show a suprafacial, stereospecific reaction pathway. The kinetics are of the first order and the whole transformation proceeds through a highly ordered cyclic transition state and is intramolecular. Crossover experiments eliminate the possibility of the rearrangement occurring via an intermolecular reaction mechanism and are consistent with an intramolecular process. There are substantial solvent effects observed in the Claisen rearrangement, where polar solvents tend to accelerate the reaction to a greater e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroboration
In organic chemistry, hydroboration refers to the addition of a hydrogen-boron bond to certain double and triple bonds involving carbon (, , , and ). This chemical reaction is useful in the organic synthesis of organic compounds. Hydroboration produces organoborane compounds that react with a variety of reagents to produce useful compounds, such as alcohols, amines, or alkyl halides. The most widely known reaction of the organoboranes is oxidation to produce alcohols typically by hydrogen peroxide. This type of reaction has promoted research on hydroboration because of its mild condition and a wide scope of tolerated alkenes. Another research subtheme is metal-catalysed hydroboration. The development of this technology and the underlying concepts were recognized by the Nobel Prize in Chemistry to Herbert C. Brown. He shared the prize with Georg Wittig in 1979 for his pioneering research on organoboranes as important synthetic intermediates. Addition of a H-B bond to C-C doubl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrocyclic Reaction
In organic chemistry, an electrocyclic reaction is a type of pericyclic rearrangement where the net result is one pi bond being converted into one sigma bond or vice versa. These reactions are usually categorized by the following criteria: * Reactions can be either photochemical or thermal. * Reactions can be either ring-opening or ring-closing (electrocyclization). * Depending on the type of reaction (photochemical or thermal) and the number of pi electrons, the reaction can happen through either a conrotatory or disrotatory mechanism. * The type of rotation determines whether the cis or trans isomer of the product will be formed. Classical examples The Nazarov cyclization reaction is a named electrocyclic reaction converting divinylketones to cyclopentenones. A classic example is the thermal ring-opening reaction of 3,4-dimethylcyclobutene. The cis isomer exclusively yields whereas the trans isomer gives the trans,trans diene: This reaction course can be explained in a sim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |