|
Hydroboration
In organic chemistry, hydroboration refers to the addition of a hydrogen- boron bond to certain double and triple bonds involving carbon (, , , and ). This chemical reaction is useful in the organic synthesis of organic compounds. Hydroboration produces organoborane compounds that react with a variety of reagents to produce useful compounds, such as alcohols, amines, or alkyl halides. The most widely known reaction of the organoboranes is oxidation to produce alcohols typically by hydrogen peroxide. This type of reaction has promoted research on hydroboration because of its mild condition and a wide scope of tolerated alkenes. Another research subtheme is metal-catalysed hydroboration. The development of this technology and the underlying concepts were recognized by the Nobel Prize in Chemistry to Herbert C. Brown. He shared the prize with Georg Wittig in 1979 for his pioneering research on organoboranes as important synthetic intermediates. Addition of a H-B bond to C-C double ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal-catalysed Hydroboration
In chemistry, metal-catalysed hydroboration is a reaction used in organic synthesis. It is one of several examples of homogeneous catalysis. History In 1975, Kono and Ito reported that Wilkinson's catalyst (Rh(PPh3)3Cl) can undergo oxidative addition with catecholborane (HBcat) or 4,4,6-trimethyl-1,3,2-dioxaborinane. These two borane compounds are otherwise slow to participate in hydroboration. In 1985, Männig and Nöth demonstrated for the first time that Wilkinson’s catalyst indeed catalyzes hydroboration of alkenes with HBcat. : Whereas uncatalyzed hydroboration using HBcat leads to reduction of the carbonyl group, the catalyzed version is selective for the alkene. : As indicated by subsequent research, transition metal-catalyzed hydroboration proceeds with attractive functional group-, regio-, stereo-, and chemo- selectivity. Mechanism The rhodium-catalyzed hydroboration reaction is thought to be initiated with the dissociation of a triphenylphosphine from the Rh(I) ce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organoborane Compound
Organoborane or organoboron compounds are chemical compounds of boron and carbon that are organic derivatives of BH3, for example trialkyl boranes. Organoboron chemistry or organoborane chemistry is the chemistry of these compounds. Organoboron compounds are important reagents in organic chemistry enabling many chemical transformations, the most important one called hydroboration. Reactions of organoborates and boranes involve the transfer of a nucleophilic group attached to boron to an electrophilic center either inter- or intramolecularly. α,β-Unsaturated borates, as well as borates with a leaving group at the α position, are highly susceptible to intramolecular 1,2-migration of a group from boron to the electrophilic α position. Oxidation or protonolysis of the resulting organoboranes may generate a variety of organic products, including alcohols, carbonyl compounds, alkenes, and halides. Properties of the B-C bond The C-B bond has low polarity (the difference in electro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the '' boron group'' it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral sodium borate, and the ultra-hard crystals of boron carbide and boron nitride. Boron is synthesized entirely by cosmic ray spallation and supernovae and not by stellar nucleosynthesis, so it is a low-abundance element in the Solar System and in the Earth's crust. It constitutes about 0.001 percent by weight of Earth's crust. It is concentrated on Earth by the water-solubility of its more common naturally occurring compounds, the borate minerals. These are mined industrially as evaporites, such as borax and kernite. The largest known deposits are in Turkey, the largest producer of boron minerals. Elemental boron is a meta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pericyclic Reaction
In organic chemistry, a pericyclic reaction is the type of organic reaction wherein the transition state of the molecule has a cyclic geometry, the reaction progresses in a concerted fashion, and the bond orbitals involved in the reaction overlap in a continuous cycle at the transition state. Pericyclic reactions stand in contrast to ''linear reactions'', encompassing most organic transformations and proceeding through an acyclic transition state, on the one hand and '' coarctate reactions'', which proceed through a doubly cyclic, concerted transition state on the other hand. Pericyclic reactions are usually rearrangement or addition reactions. The major classes of pericyclic reactions are given in the table below (the three most important classes are shown in bold). Ene reactions and cheletropic reactions are often classed as group transfer reactions and cycloadditions/cycloeliminations, respectively, while dyotropic reactions and group transfer reactions (if ene reactions are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Georg Wittig
Georg Wittig (; 16 June 1897 – 26 August 1987) was a German chemist who reported a method for synthesis of alkenes from aldehydes and ketones using compounds called phosphonium ylides in the Wittig reaction. He shared the Nobel Prize in Chemistry with Herbert C. Brown in 1979. Biography Wittig was born in Berlin, Germany and shortly after his birth moved with his family to Kassel, where his father was professor at the applied arts high school. He attended school in Kassel and started studying chemistry at the University of Tübingen 1916. He was drafted and became a lieutenant in the cavalry of Hesse-Kassel (or Hesse-Cassel). After being an Allied prisoner of war from 1918 until 1919, Wittig found it hard to restart his chemistry studies owing to overcrowding at the universities. By a direct plea to Karl von Auwers, who was professor for organic chemistry at the University of Marburg at the time, he was able to resume university study and after 3 years was awarded the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nobel Prize In Chemistry
) , image = Nobel Prize.png , alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "MDCCCXXXIII" above, followed by (smaller) "OB•" then "MDCCCXCVI" below. , awarded_for = Outstanding contributions in chemistry , presenter = Royal Swedish Academy of Sciences , location = Stockholm, Sweden , reward = 9 million SEK (2017) , year = 1901 , holder = Carolyn R. Bertozzi, Morten P. Meldal and Karl Barry Sharpless (2022) , most_awards = Frederick Sanger and Karl Barry Sharpless (2) , website nobelprize.org, previous = 2021 , year2=2022, main= 2022, next= 2023 The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, award ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Herbert C
Herbert may refer to: People Individuals * Herbert (musician), a pseudonym of Matthew Herbert Name * Herbert (given name) * Herbert (surname) Places Antarctica * Herbert Mountains, Coats Land * Herbert Sound, Graham Land Australia * Herbert, Northern Territory, a rural locality * Herbert, South Australia. former government town * Division of Herbert, an electoral district in Queensland * Herbert River, a river in Queensland * County of Herbert, a cadastral unit in South Australia Canada * Herbert, Saskatchewan, Canada, a town * Herbert Road, St. Albert, Canada New Zealand * Herbert, New Zealand The small town of Herbert, formerly Otepopo, lies in North Otago, New Zealand, north of Dunedin and south-west of Oamaru. It lies on the edge of the Herbert Forest. Herbert consists of a group of houses and three churches clustered around St ..., a town * Mount Herbert (New Zealand) United States * Herbert, Illinois, an unincorporated community * Herbert, Michigan, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes Physical property, physical and Chemical property, chemical properties, and evaluation of Reactivity (chemistry), chemical reactivity to understand their behavior. The study of organic reactions includes the organic synthesis, chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical (in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Markovnikov's Rule
In organic chemistry, Markovnikov's rule or Markownikoff's rule describes the outcome of some addition reactions. The rule was formulated by Russian chemist Vladimir Markovnikov in 1870. Explanation The rule states that with the addition of a protic acid HX or other polar reagent to an asymmetric alkene, the acid hydrogen (H) or electropositive part gets attached to the carbon with more hydrogen substituents, and the halide (X) group or electronegative part gets attached to the carbon with more alkyl substituents. This is in contrast to Markovnikov's original definition, in which the rule is stated that the X component is added to the carbon with the fewest hydrogen atoms while the hydrogen atom is added to the carbon with the greatest number of hydrogen atoms. The same is true when an alkene reacts with water in an addition reaction to form an alcohol which involve formation of carbocations. The hydroxyl group (OH) bonds to the carbon that has the greater number of carbon–c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
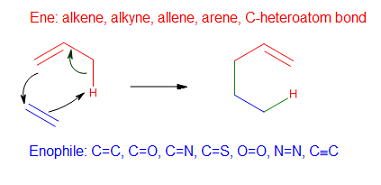
Group Transfer Reaction
In organic chemistry, a group transfer reaction is a pericyclic process where one or more groups of atoms is transferred from one molecule to another. They can sometimes be difficult to identify when separate reactant molecules combine into a single product molecule (like in the ene reaction). Unlike other pericyclic reaction classes, group transfer reactions do not have a specific conversion of pi bonds into sigma bonds or vice versa, and tend to be less frequently encountered. Like all pericyclic reactions, they must obey the Woodward–Hoffmann rules. The best known group transfer reaction is the ene reaction In organic chemistry, the ene reaction (also known as the Alder-ene reaction by its discoverer Kurt Alder in 1943) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile ... in which an allylic hydrogen is transferred to an alkene. References Rearrangement reactions Pericyclic reactions< ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |