|
ZINDO
ZINDO is a semi-empirical quantum chemistry method used in computational chemistry. It is a development of the INDO method. It stands for Zerner's Intermediate Neglect of Differential Overlap, as it was developed by Michael Zerner and his coworkers in the 1970s. Unlike INDO, which was really restricted to organic molecules and those containing the atoms B to F, ZINDO covers a wide range of the periodic table, even including the rare-earth elements. There are two distinct versions of the method: * ZINDO/1 – for calculating ground-state properties such as bond lengths and bond angles. It refers to a SCF (RHF or ROHF) calculation with the INDO/1 level as suggested by Pople, which provides the reference state MO coefficients. Ground-state dipole moments and ionization potentials are in general very accurate. Geometry optimizations are erratic, what prompted Zerner's group to improve the performance of the code in the late 1990sJ. D. Da Motta Neto, M. Zerner, Int. J. Quantum Chem. 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Michael Zerner
Michael Charles Zerner (January 1, 1940 – February 2, 2000) was an American theoretical chemist, professor at the University of Guelph from 1970 to 1981 and University of Florida from 1981 to 2000. Zerner earned his Ph.D. under Martin Gouterman Martin Paul Gouterman (December 26, 1931 – February 22, 2020) was an American chemist who was a Professor of Chemistry at the University of Washington. He is remembered for his seminal work on the optical spectra porphyrins, for which he develo ... at Harvard, working with the spectroscopy of porphyrins. He conceived and wrote a quantum chemistry program, known as BIGSPEC or ZINDO, for calculating electronic spectra of big molecules. In 1996 Zerner was diagnosed with liver cancer, and died on February 2, 2000, survived by his wife and two children. External links * * 1940 births 2000 deaths University of Florida faculty Theoretical chemists Harvard University alumni Computational chemists Academic staff of the Uni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semi-empirical Quantum Chemistry Methods
Semi-empirical quantum chemistry methods are based on the Hartree–Fock formalism, but make many approximations and obtain some parameters from empirical data. They are very important in computational chemistry for treating large molecules where the full Hartree–Fock method without the approximations is too expensive. The use of empirical parameters appears to allow some inclusion of electron correlation effects into the methods. Within the framework of Hartree–Fock calculations, some pieces of information (such as two-electron integrals) are sometimes approximated or completely omitted. In order to correct for this loss, semi-empirical methods are parametrized, that is their results are fitted by a set of parameters, normally in such a way as to produce results that best agree with experimental data, but sometimes to agree with ''ab initio'' results. Type of simplifications used Semi-empirical methods follow what are often called empirical methods where the two-electron p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Computational Chemistry
Computational chemistry is a branch of chemistry that uses computer simulation to assist in solving chemical problems. It uses methods of theoretical chemistry, incorporated into computer programs, to calculate the structures and properties of molecules, groups of molecules, and solids. It is essential because, apart from relatively recent results concerning the hydrogen molecular ion ( dihydrogen cation, see references therein for more details), the quantum many-body problem cannot be solved analytically, much less in closed form. While computational results normally complement the information obtained by chemical experiments, it can in some cases predict hitherto unobserved chemical phenomena. It is widely used in the design of new drugs and materials. Examples of such properties are structure (i.e., the expected positions of the constituent atoms), absolute and relative (interaction) energies, electronic charge density distributions, dipoles and higher multipole moments ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
INDO
Indo may refer to: * Indo-, a prefix indicating India or the Indian Subcontinent * Indonesia, a country in Asia ** INDO LINES, callsign of Indonesian Airlines ** Indo people, people of mixed European and Indonesian ancestry ** Indo cuisine, fusion cuisine of Indonesian and European * INDO, the Intermediate Neglect of Differential Overlap semi-empirical method * Indo (apple), an apple cultivar * Irish Independent, commonly nicknamed 'The Indo' * The slang term 'endo' or 'indo' is an AAVE African-American Vernacular English (AAVE, ), also referred to as Black (Vernacular) English, Black English Vernacular, or occasionally Ebonics (a colloquial, controversial term), is the variety of English natively spoken, particularly in urba ... prounuciation for " indoor"-grown marijuana. * Palacio de Indo, a former palace in Madrid See also * Endo (other) * {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reviews In Computational Chemistry
A review is an evaluation of a publication, product, service, or company or a critical take on current affairs in literature, politics or culture. In addition to a critical evaluation, the review's author may assign the work a rating to indicate its relative merit. A compilation of reviews may itself be called a review. Reviews can apply to a movie (a movie review), video game (video game review), musical composition ( music review of a composition or recording), book (book review); a piece of hardware like a car, home appliance, or computer; or software such as business software, sales software; or an event or performance, such as a live music concert, play, musical theater show, dance show or art exhibition In the cultural sphere, ''The New York Review of Books'', for instance, is a collection of essays on literature, culture, and current affairs. ''National Review'', founded by William F. Buckley Jr., is a conservative magazine, and ''Monthly Review'' is a long-run ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the '' boron group'' it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral sodium borate, and the ultra-hard crystals of boron carbide and boron nitride. Boron is synthesized entirely by cosmic ray spallation and supernovae and not by stellar nucleosynthesis, so it is a low-abundance element in the Solar System and in the Earth's crust. It constitutes about 0.001 percent by weight of Earth's crust. It is concentrated on Earth by the water-solubility of its more common naturally occurring compounds, the borate minerals. These are mined industrially as evaporites, such as borax and kernite. The largest known deposits are in Turkey, the largest producer of boron minerals. Elemental boron is a meta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactive, as it reacts with all other elements except for the light inert gases. Among the elements, fluorine ranks 24th in universal abundance and 13th in terrestrial abundance. Fluorite, the primary mineral source of fluorine which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning 'flow' gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist Henri Moissan isolate elemental fluorine using low-temperature electrolysis, a process still employed for moder ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Periodic Table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of chemistry. It is a graphic formulation of the periodic law, which states that the properties of the chemical elements exhibit an approximate periodic dependence on their atomic numbers. The table is divided into four roughly rectangular areas called blocks. The rows of the table are called periods, and the columns are called groups. Elements from the same group of the periodic table show similar chemical characteristics. Trends run through the periodic table, with nonmetallic character (keeping their own electrons) increasing from left to right across a period, and from down to up across a group, and metallic character (surrendering electrons to other atoms) increasing in the opposite direction. The underlying reason for these trend ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rare-earth Element
The rare-earth elements (REE), also called the rare-earth metals or (in context) rare-earth oxides or sometimes the lanthanides (yttrium and scandium are usually included as rare earths), are a set of 17 nearly-indistinguishable lustrous silvery-white soft heavy metals. Compounds containing rare earths have diverse applications in electrical and electronic components, lasers, glass, magnetic materials, and industrial processes. Scandium and yttrium are considered rare-earth elements because they tend to occur in the same ore deposits as the lanthanides and exhibit similar chemical properties, but have different electronic and magnetic properties. These metals tarnish slowly in air at room temperature and react slowly with cold water to form hydroxides, liberating hydrogen. They react with steam to form oxides, and at elevated temperature (400°C) ignite spontaneously. These elements and their compounds have no biological function other than in several specialized enzymes, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
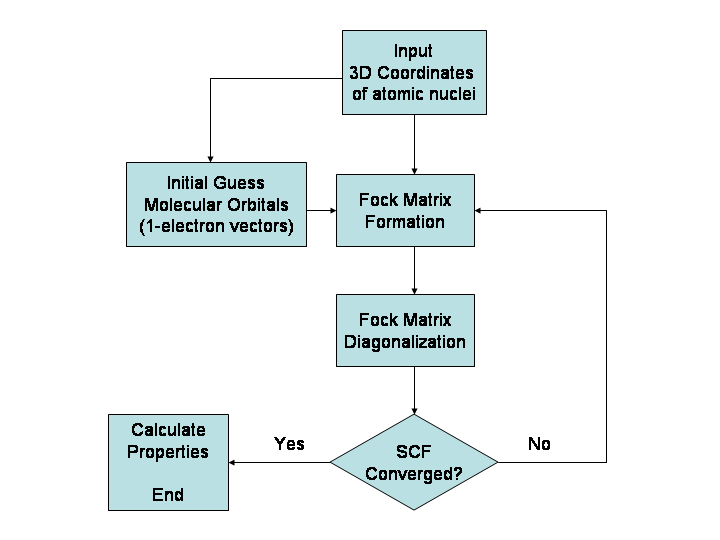
Hartree–Fock Method
In computational physics and chemistry, the Hartree–Fock (HF) method is a method of approximation for the determination of the wave function and the energy of a quantum many-body system in a stationary state. The Hartree–Fock method often assumes that the exact ''N''-body wave function of the system can be approximated by a single Slater determinant (in the case where the particles are fermions) or by a single permanent (in the case of bosons) of ''N'' spin-orbitals. By invoking the variational method, one can derive a set of ''N''-coupled equations for the ''N'' spin orbitals. A solution of these equations yields the Hartree–Fock wave function and energy of the system. Especially in the older literature, the Hartree–Fock method is also called the self-consistent field method (SCF). In deriving what is now called the Hartree equation as an approximate solution of the Schrödinger equation, Hartree required the final field as computed from the charge distributi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
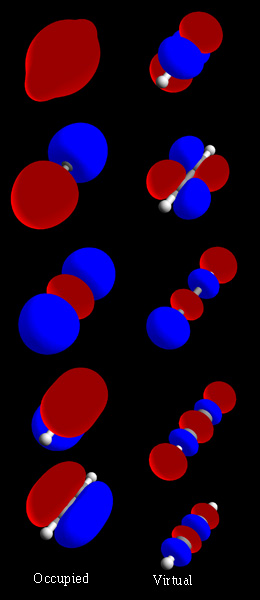
Molecular Orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The terms ''atomic orbital'' and ''molecular orbital'' were introduced by Robert S. Mulliken in 1932 to mean ''one-electron orbital wave functions''. At an elementary level, they are used to describe the ''region'' of space in which a function has a significant amplitude. In an isolated atom, the orbital electrons' location is determined by functions called atomic orbitals. When multiple atoms combine chemically into a molecule, the electrons' locations are determined by the molecule as a whole, so the atomic orbitals combine to form molecular orbitals. The electrons from the constituent atoms occupy the molecular orbitals. Mathematically, molecular orbitals are an approximate solution to the Schrö ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Configuration Interaction
Configuration interaction (CI) is a post-Hartree–Fock linear variational method for solving the nonrelativistic Schrödinger equation within the Born–Oppenheimer approximation for a quantum chemical multi-electron system. Mathematically, ''configuration'' simply describes the linear combination of Slater determinants used for the wave function. In terms of a specification of orbital occupation (for instance, (1s)2(2s)2(2p)1...), ''interaction'' means the mixing (interaction) of different electronic configurations (states). Due to the long CPU time and large memory required for CI calculations, the method is limited to relatively small systems. In contrast to the Hartree–Fock method, in order to account for electron correlation, CI uses a variational wave function that is a linear combination of configuration state functions (CSFs) built from spin orbitals (denoted by the superscript ''SO''), : \Psi = \sum_ c_ \Phi_^ = c_0\Phi_0^ + c_1\Phi_1^ + where ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |