|
Yttrium Borides
Yttrium boride refers to a crystalline material composed of different proportions of yttrium and boron, such as YB2, YB4, YB6, YB12, YB25, YB50 and YB66. They are all gray-colored, hard solids having high melting temperatures. The most common form is the yttrium hexaboride YB6. It exhibits superconductivity at relatively high temperature of 8.4 K and, similar to LaB6, is an electron cathode. Another remarkable yttrium boride is YB66. It has a large lattice constant (2.344 nm), high thermal and mechanical stability, and therefore is used as a diffraction grating for low-energy synchrotron radiation (1–2 keV). YB2 (yttrium diboride) Yttrium diboride has the same hexagonal crystal structure as aluminium diboride and magnesium diboride – an important superconducting material. Its Pearson symbol is ''hP3'', space group P6/mmm (No 191), ''a'' = 0.33041 nm, ''c'' = 0.38465 nm and the calculated density is 5.05 g/cm3. In this structure, the boron atoms form graphite lik ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pearson Symbol
The Pearson symbol, or Pearson notation, is used in crystallography as a means of describing a crystal structure, and was originated by W. B. Pearson. The symbol is made up of two letters followed by a number. For example: * Diamond structure, ''cF''8 * Rutile structure, ''tP''6 The two (italicised) letters specify the Bravais lattice. The lower-case letter specifies the crystal family, and the upper-case letter the centering type. The number at the end of the Pearson symbol gives the number of the atoms in the conventional unit cell.Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005 IR-3.4.4, pp. 49–51; IR-11.5, pp. 241–242. |
Lanthanum Hexaboride
Lanthanum hexaboride ( La B6, also called lanthanum boride and LaB) is an inorganic chemical, a boride of lanthanum. It is a refractory ceramic material that has a melting point of 2210 °C, and is insoluble in water and hydrochloric acid. It is extremely hard, with a Mohs hardness of 9.5. It has a low work function and one of the highest electron emissivities known, and is stable in vacuum. Stoichiometric samples are colored intense purple-violet, while boron-rich ones (above LaB6.07) are blue. Ion bombardment changes its color from purple to emerald green. LaB6 is a superconductor with a relatively low transition temperature of 0.45 K. Uses The principal use of lanthanum hexaboride is in hot cathodes, either as a single crystal or as a coating deposited by physical vapor deposition. Hexaborides, such as lanthanum hexaboride (LaB6) and cerium hexaboride (CeB6), have low work functions, around 2.5 eV. They are also somewhat resistant to cathode poisoning. Cerium h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
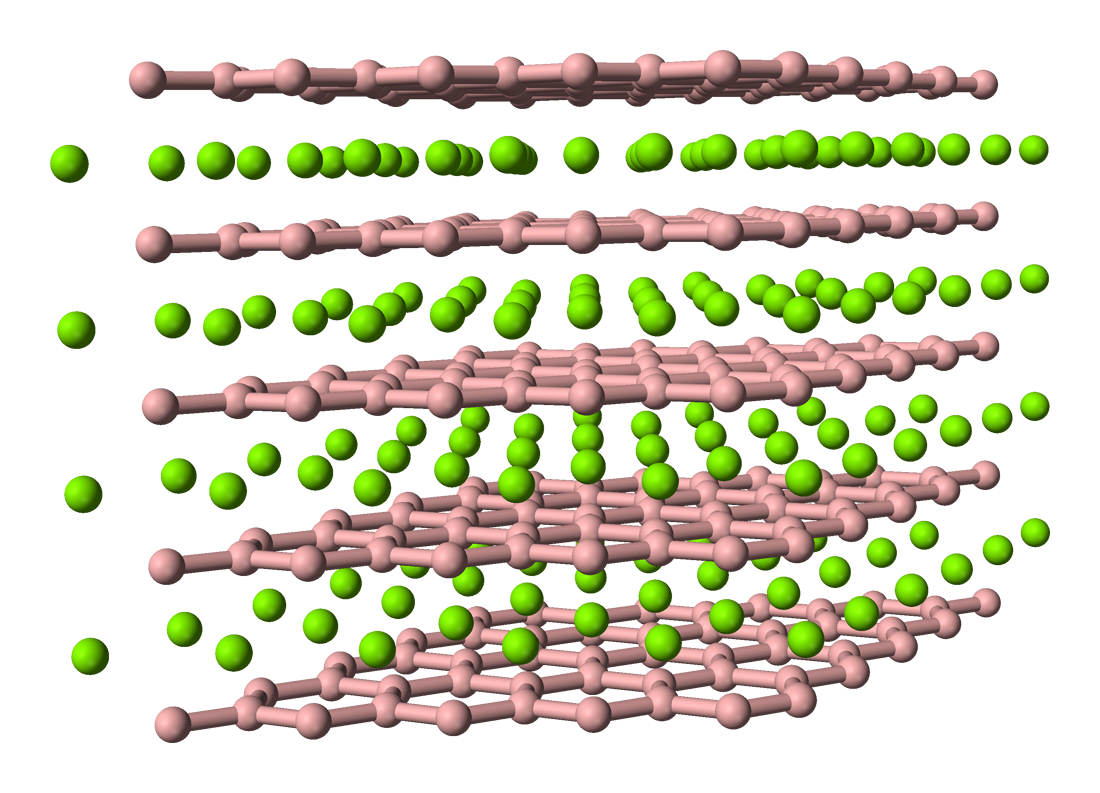
Crystal Structure Of Boron-rich Metal Borides
Metals, and specifically rare-earth elements, form numerous chemical complexes with boron. Their crystal structure and chemical bonding depend strongly on the metal element M and on its atomic ratio to boron. When B/M ratio exceeds 12, boron atoms form B12 icosahedra which are linked into a three-dimensional boron framework, and the metal atoms reside in the voids of this framework. Those icosahedra are basic structural units of most allotropes of boron and boron-rich rare-earth borides. In such borides, metal atoms donate electrons to the boron polyhedra, and thus these compounds are regarded as electron-deficient solids. The crystal structures of many boron-rich borides can be attributed to certain types including MgAlB14, YB66, REB41Si1.2, B4C and other, more complex types such as RExB12C0.33Si3.0. Some of these formulas, for example B4C, YB66 and MgAlB14, historically reflect the idealistic structures, whereas the experimentally determined composition is nonstoichiometric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Knoop Hardness
The Knoop hardness test is a microhardness test – a test for mechanical hardness used particularly for very brittle materials or thin sheets, where only a small indentation may be made for testing purposes. A pyramidal diamond point is pressed into the polished surface of the test material with a known (often 100g) load, for a specified dwell time, and the resulting indentation is measured using a microscope. The geometry of this indenter is an extended pyramid with the length to width ratio being 7:1 and respective face angles are 172 degrees for the long edge and 130 degrees for the short edge. The depth of the indentation can be approximated as 1/30 of the long dimension. The Knoop hardness ''HK'' or ''KHN'' is then given by the formula: :HK where: :''L'' = length of indentation along its long axis :''C''p = correction factor related to the shape of the indenter, ideally 0.070279 :''P'' = load HK values are typically in the range from 100 to 1000, when specified in the co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cubic Crystal System
In crystallography, the cubic (or isometric) crystal system is a crystal system where the Crystal_structure#Unit_cell, unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals. There are three main varieties of these crystals: *Primitive cubic (abbreviated ''cP'' and alternatively called simple cubic) *Body-centered cubic (abbreviated ''cI'' or bcc) *Face-centered cubic (abbreviated ''cF'' or fcc, and alternatively called Close-packing_of_equal_spheres, ''cubic close-packed'' or ccp) Each is subdivided into other variants listed below. Although the ''unit cells'' in these crystals are conventionally taken to be cubes, the primitive_cell, primitive unit cells often are not. Bravais lattices The three Bravais lattices in the cubic crystal system are: The primitive cubic lattice (cP) consists of one Lattice_(group), lattice point on each corner of the cube; this means each simple cubic unit cell has in total one latt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a rectangular base (''a'' by ''b'') and height (''c''), such that ''a'', ''b'', and ''c'' are distinct. All three bases intersect at 90° angles, so the three lattice vectors remain mutually orthogonal. Bravais lattices There are four orthorhombic Bravais lattices: primitive orthorhombic, base-centered orthorhombic, body-centered orthorhombic, and face-centered orthorhombic. For the base-centered orthorhombic lattice, the primitive cell has the shape of a right rhombic prism;See , row oC, column Primitive, where the cell parameters are given as a1 = a2, α = β = 90° it can be constructed because the two-dimensional centered rectangular base layer can also be described with primitive rhombic axes. Note that the length a of the primit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yttria
Yttrium oxide, also known as yttria, is Y2 O3. It is an air-stable, white solid substance. The thermal conductivity of yttrium oxide is 27 W/(m·K). Uses Phosphors Yttria is widely used to make Eu:YVO4 and Eu:Y2O3 phosphors that give the red color in color TV picture tubes. Yttria lasers Y2O3 is a prospective solid-state laser material. In particular, lasers with ytterbium as dopant allow the efficient operation both in continuous operation and in pulsed regimes. At high concentration of excitations (of order of 1%) and poor cooling, the quenching of emission at laser frequency and avalanche broadband emission takes place. (Yttria-based lasers are not to be confused with YAG lasers using yttrium aluminum garnet, a widely used crystal host for rare earth laser dopants). Gas Lighting The original use of the mineral yttria and the purpose of its extraction from mineral sources was as part of the process of making gas mantles and other products for turning the flames of artifici ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Icosahedron
In geometry, an icosahedron ( or ) is a polyhedron with 20 faces. The name comes and . The plural can be either "icosahedra" () or "icosahedrons". There are infinitely many non- similar shapes of icosahedra, some of them being more symmetrical than others. The best known is the (convex, non- stellated) regular icosahedron—one of the Platonic solids—whose faces are 20 equilateral triangles. Regular icosahedra There are two objects, one convex and one nonconvex, that can both be called regular icosahedra. Each has 30 edges and 20 equilateral triangle faces with five meeting at each of its twelve vertices. Both have icosahedral symmetry. The term "regular icosahedron" generally refers to the convex variety, while the nonconvex form is called a ''great icosahedron''. Convex regular icosahedron The convex regular icosahedron is usually referred to simply as the ''regular icosahedron'', one of the five regular Platonic solids, and is represented by its Schläfli symbol , con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Debye Temperature
In thermodynamics and solid-state physics, the Debye model is a method developed by Peter Debye in 1912 for estimating the phonon contribution to the specific heat (Heat capacity) in a solid. It treats the vibrations of the atomic lattice (heat) as phonons in a box, in contrast to the Einstein photoelectron model, which treats the solid as many individual, non-interacting quantum harmonic oscillators. The Debye model correctly predicts the low-temperature dependence of the heat capacity of solids, which is proportional to T^3 – the Debye ''T'' 3 law. Just like the Einstein photoelectron model, it also recovers the Dulong–Petit law at high temperatures. But due to simplifying assumptions, its accuracy suffers at intermediate temperatures. Derivation The Debye model is a solid-state equivalent of Planck's law of black body photon radiation, where one treats electromagnetic photonic radiation as a photon gas. The Debye model treats atomic vibrations as phonons in a box ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |