|
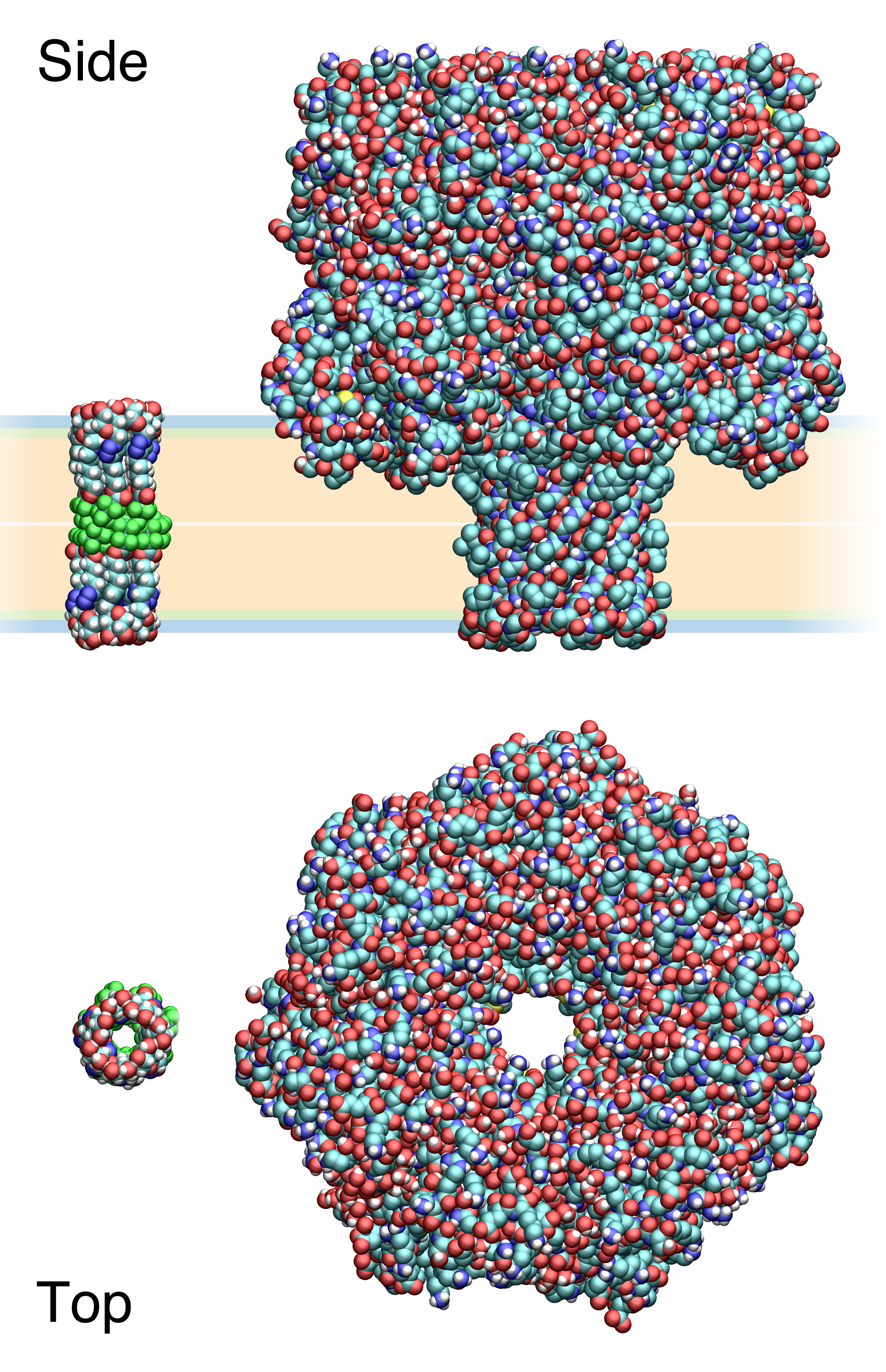
Synthetic Ion Channels
Synthetic ion channels are ''de novo'' chemical compounds that insert into lipid bilayers, form pores, and allow ions to flow from one side to the other. They are man-made analogues of natural ion channels, and are thus also known as artificial ion channels. Compared to biological channels, they usually allow fluxes of similar magnitude but are # minuscule in size (less than 5k Dalton vs. > 100k Dalton), # diverse in molecular architecture, and # may rely on diverse supramolecular interactions to pre-form the active, conducting structures. Synthetic channels, like natural channels, are usually characterized by a combination of single-molecule (e.g., voltage-clamp of planar bilayers) and ensemble techniques (flux in vesicles). The study of synthetic ion channels can potentially lead to new single-molecule sensing technologies as well as new therapeutics. History While semi-synthetic ion channels, often based on modified peptidic channels like gramicidin, had been prepare ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Passive Transport
Passive transport is a type of membrane transport that does not require energy to move substances across cell membranes. Instead of using cellular energy, like active transport, passive transport relies on the second law of thermodynamics to drive the movement of substances across cell membranes. Fundamentally, substances follow Fick's first law, and move from an area of high concentration to one of low concentration because this movement increases the entropy of the overall system. The rate of passive transport depends on the permeability of the cell membrane, which, in turn, depends on the organization and characteristics of the membrane lipids and proteins. The four main kinds of passive transport are simple diffusion, facilitated diffusion, filtration, and/or osmosis. Passive transport follows Fick's first law and the second law of thermodynamics. Diffusion Diffusion is the net movement of material from an area of high concentration to an area with lower concentration ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Picoampere
The ampere (, ; symbol: A), often shortened to amp,SI supports only the use of symbols and deprecates the use of abbreviations for units. is the unit of electric current in the International System of Units (SI). One ampere is equal to electrons worth of charge moving past a point in a second. It is named after French mathematician and physicist André-Marie Ampère (1775–1836), considered the father of electromagnetism along with Danish physicist Hans Christian Ørsted. As of the 2019 redefinition of the SI base units, the ampere is defined by fixing the elementary charge to be exactly C (coulomb), which means an ampere is an electrical current equivalent to elementary charges moving every seconds or elementary charges moving in a second. Prior to the redefinition the ampere was defined as the current that would need to be passed through 2 parallel wires 1 metre apart to produce a magnetic force of newtons per metre. The earlier CGS system had two definitions of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capacitance
Capacitance is the capability of a material object or device to store electric charge. It is measured by the change in charge in response to a difference in electric potential, expressed as the ratio of those quantities. Commonly recognized are two closely related notions of capacitance: ''self capacitance'' and ''mutual capacitance''. An object that can be electrically charged exhibits self capacitance, for which the electric potential is measured between the object and ground. Mutual capacitance is measured between two components, and is particularly important in the operations of the capacitor, a device designed for this purpose as an elementary Linear circuit, linear electronic component. Capacitance is a function only of the geometry of the design of the capacitor, e.g., the opposing surface area of the plates and the distance between them, and the permittivity of the dielectric material between the plates. For many dielectric materials, the permittivity and thus the capaci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved. Electrically, such a solution is neutral. If an electric potential is applied to such a solution, the cations of the solution are drawn to the electrode that has an abundance of electrons, while the anions are drawn to the electrode that has a deficit of electrons. The movement of anions and cations in opposite directions within the solution amounts to a current. Some gases, such as hydrogen chloride (HCl), under conditions of high temperature or low pressure can also function as electrolytes. El ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Energy Barrier
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). Activation energy can be thought of as the magnitude of the potential barrier (sometimes called the energy barrier) separating minima of the potential energy surface pertaining to the initial and final thermodynamic state. For a chemical reaction to proceed at a reasonable rate, the temperature of the system should be high enough such that there exists an appreciable number of molecules with translational energy equal to or greater than the activation energy. The term "activation energy" was introduced in 1889 by the Swedish scientist Svante Arrhenius. Other uses Although less commonly used, activation energy also applies to nuclear reactions and various other physical phenomena. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescence Spectroscopy
Fluorescence spectroscopy (also known as fluorimetry or spectrofluorometry) is a type of electromagnetic spectroscopy that analyzes fluorescence from a sample. It involves using a beam of light, usually ultraviolet light, that excites the electrons in molecules of certain compounds and causes them to emit light; typically, but not necessarily, visible light. A complementary technique is absorption spectroscopy. In the special case of single molecule fluorescence spectroscopy, intensity fluctuations from the emitted light are measured from either single fluorophores, or pairs of fluorophores. Devices that measure fluorescence are called fluorometers. Theory Molecules have various states referred to as energy levels. Fluorescence spectroscopy is primarily concerned with electronic and vibrational states. Generally, the species being examined has a ground electronic state (a low energy state) of interest, and an excited electronic state of higher energy. Within each of these elec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
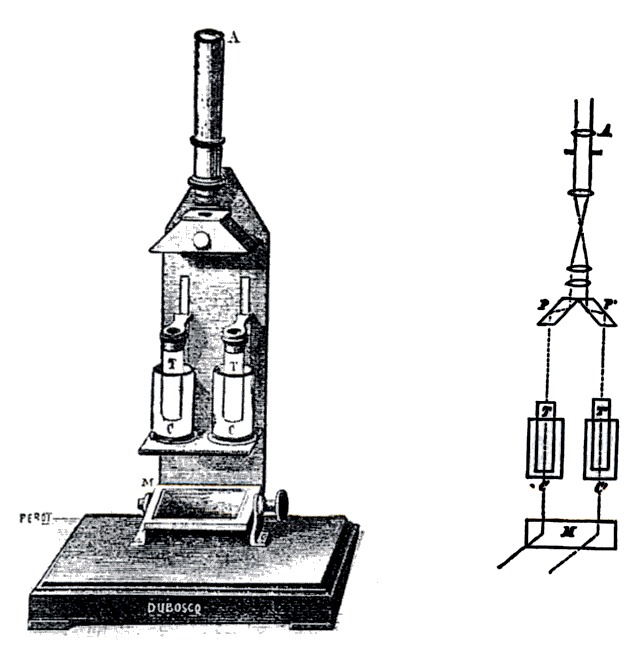
Colorimetry (chemical Method)
In physical and analytical chemistry, colorimetry or colourimetry is a technique used to determine the concentration of colored compounds in solution. A colorimeter is a device used to test the concentration of a solution by measuring its absorbance of a specific wavelength of light (not to be confused with the tristimulus colorimeter used to measure colors in general). To use the colorimeter, different solutions must be made, including a control or reference of known concentration. With a visual colorimeter, for example the Duboscq colorimeter illustrated, the length of the light path through the solutions can be varied while filtered light transmitted through them is compared for a visual match. The concentration times path length is taken to be equal when the colors match, so the concentration of the unknown can be determined by simple proportions. Nessler tubes work on the same principle. There are also electronic automated colorimeters; before these machines are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectroscopy
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter waves and acoustic waves can also be considered forms of radiative energy, and recently gravitational waves have been associated with a spectral signature in the context of the Laser Interferometer Gravitational-Wave Observatory (LIGO) In simpler terms, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum. Historically, spectroscopy originated as the study of the wavelength dependence of the absorption by gas phase matter of visible light dispersed by a prism. Spectroscopy, primarily in the electromagnetic spectrum, is a fundamental exploratory tool in the fields of astronomy, chemistry, materials science, and physics, allowing the composition, physical structure and e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Statistical Ensemble
In physics, specifically statistical mechanics, an ensemble (also statistical ensemble) is an idealization consisting of a large number of virtual copies (sometimes infinitely many) of a system, considered all at once, each of which represents a possible state that the real system might be in. In other words, a statistical ensemble is a set of systems of particles used in statistical mechanics to describe a single system. The concept of an ensemble was introduced by J. Willard Gibbs in 1902. A thermodynamic ensemble is a specific variety of statistical ensemble that, among other properties, is in statistical equilibrium (defined below), and is used to derive the properties of thermodynamic systems from the laws of classical or quantum mechanics. Physical considerations The ensemble formalises the notion that an experimenter repeating an experiment again and again under the same macroscopic conditions, but unable to control the microscopic details, may expect to observe a rang ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Macroscopic Scale
The macroscopic scale is the length scale on which objects or phenomena are large enough to be visible with the naked eye, without magnifying optical instruments. It is the opposite of microscopic. Overview When applied to physical phenomena and bodies, the macroscopic scale describes things as a person can directly perceive them, without the aid of magnifying devices. This is in contrast to observations (microscopy) or theories ( microphysics, statistical physics) of objects of geometric lengths smaller than perhaps some hundreds of micrometers. A macroscopic view of a ball is just that: a ball. A microscopic view could reveal a thick round skin seemingly composed entirely of puckered cracks and fissures (as viewed through a microscope) or, further down in scale, a collection of molecules in a roughly spherical shape (as viewed through an electron microscope). An example of a physical theory that takes a deliberately macroscopic viewpoint is thermodynamics. An example of a topi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |