|
Electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved. Electrically, such a solution is neutral. If an electric potential is applied to such a solution, the cations of the solution are drawn to the electrode that has an abundance of electrons, while the anions are drawn to the electrode that has a deficit of electrons. The movement of anions and cations in opposite directions within the solution amounts to a current. Some gases, such as hydrogen chloride (HCl), under conditions of high temperature or low pressure can also function as elect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
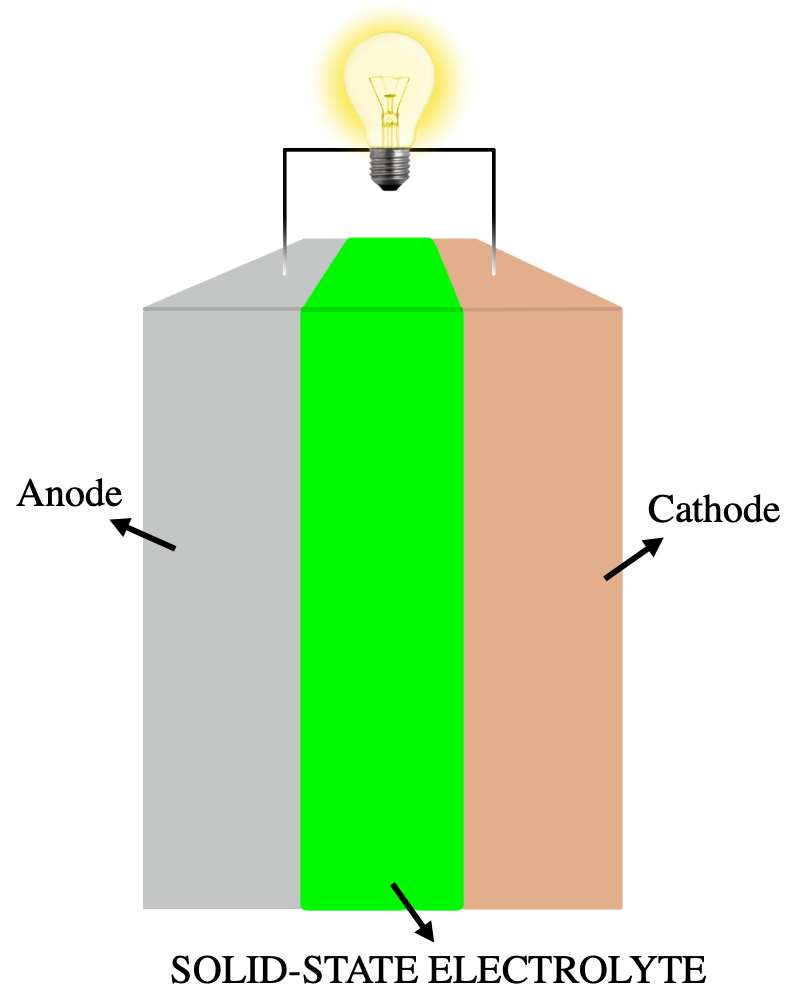
Solid-state Electrolyte
A solid-state electrolyte (SSE) is a solid ionic conductor and electron-insulating material and it is the characteristic component of the solid-state battery. It is useful for applications in electrical energy storage (EES) in substitution of the liquid electrolytes found in particular in lithium-ion battery. The main advantages are the absolute safety, no issues of leakages of toxic organic solvents, low flammability, non-volatility, mechanical and thermal stability, easy processability, low self-discharge, higher achievable power density and cyclability. This makes possible, for example, the use of a lithium metal anode in a practical device, without the intrinsic limitations of a liquid electrolyte thanks to the property of lithium dendrite suppression in the presence of a solid-state electrolyte membrane. The utilization of a high capacity anode and low reduction potential, like lithium with a specific capacity of 3860 mAh g−1 and a reduction potential of -3.04 V vs SHE, in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyelectrolyte
Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Polycations and polyanions are polyelectrolytes. These groups dissociate in aqueous solutions (water), making the polymers charged. Polyelectrolyte properties are thus similar to both electrolytes (salts) and polymers (high molecular weight compounds) and are sometimes called polysalts. Like salts, their solutions are electrically conductive. Like polymers, their solutions are often viscous. Charged molecular chains, commonly present in soft matter systems, play a fundamental role in determining structure, stability and the interactions of various molecular assemblies. Theoretical approaches to describing their statistical properties differ profoundly from those of their electrically neutral counterparts, while technological and industrial fields exploit their unique properties. Many biological molecules are polyelectrolytes. For instance, polypeptides, glycosaminoglycans, and DNA are polyelectrolytes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conductivity (electrolytic)
Conductivity (or specific conductance) of an electrolyte solution is a measure of its ability to conduct electricity. The SI unit of conductivity is Siemens per meter (S/m). Conductivity measurements are used routinely in many industrial and environmental applications as a fast, inexpensive and reliable way of measuring the ionic content in a solution. For example, the measurement of product conductivity is a typical way to monitor and continuously trend the performance of water purification systems. In many cases, conductivity is linked directly to the total dissolved solids (TDS). High quality deionized water has a conductivity of about 0.05 μS/cm at 25 °C, typical drinking water is in the range of 200–800 μS/cm, while sea water is about 50 mS/cm ncorrect according to source(or 50,000 μS/cm). Conductivity is traditionally determined by connecting the electrolyte in a Wheatstone bridge. Dilute solutions follow Kohlrausch's Laws of conc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
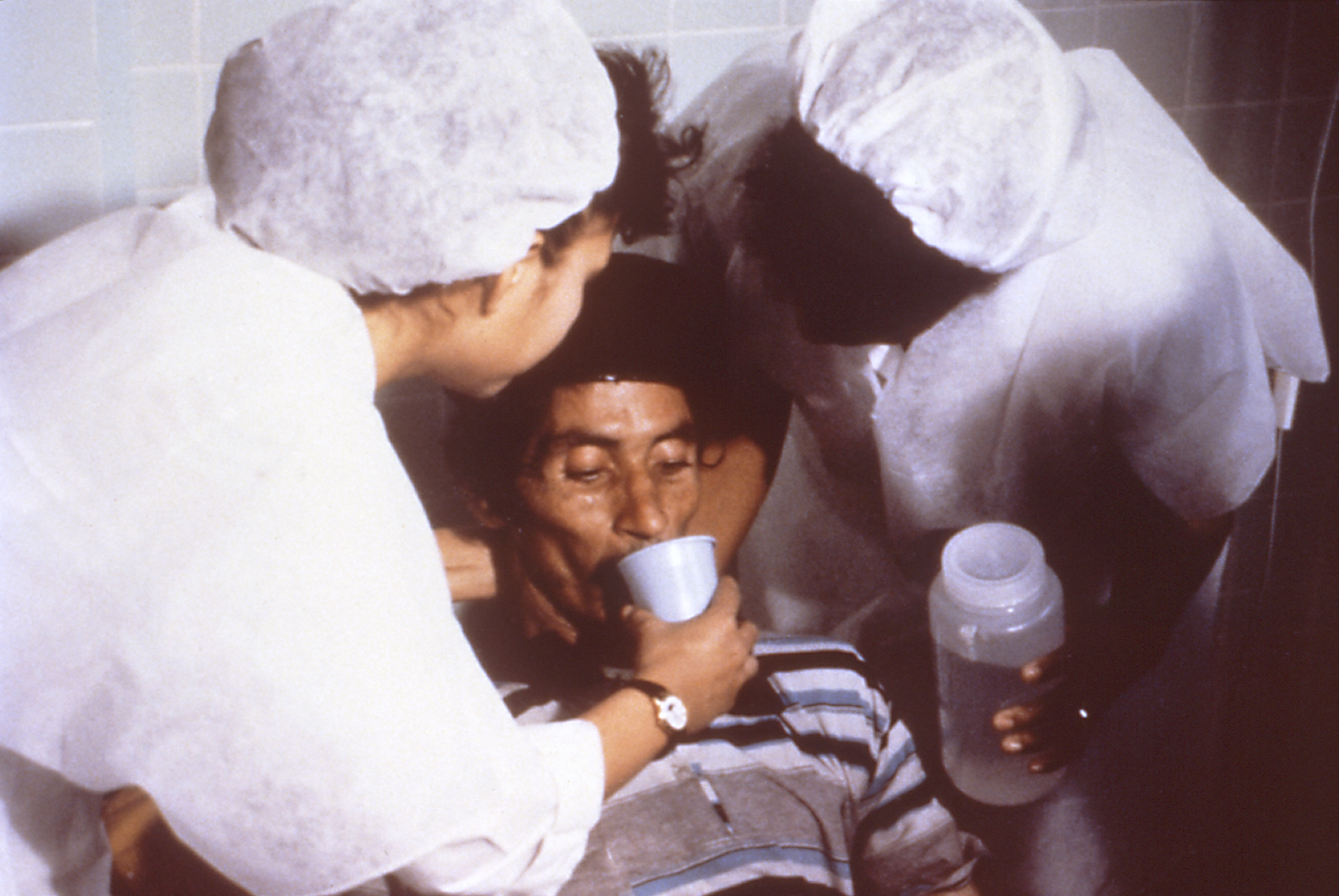
Oral Rehydration Therapy
Oral rehydration therapy (ORT) is a type of fluid replacement used to prevent and treat dehydration, especially due to diarrhea. It involves drinking water with modest amounts of sugar and salts, specifically sodium and potassium. Oral rehydration therapy can also be given by a nasogastric tube. Therapy should routinely include the use of zinc supplements. Use of oral rehydration therapy has been estimated to decrease the risk of death from diarrhea by up to 93%. Side effects may include vomiting, high blood sodium, or high blood potassium. If vomiting occurs, it is recommended that use be paused for 10 minutes and then gradually restarted. The recommended formulation includes sodium chloride, sodium citrate, potassium chloride, and glucose. Glucose may be replaced by sucrose and sodium citrate may be replaced by sodium bicarbonate, if not available. It works as glucose increases the uptake of sodium and thus water by the intestines. A number of other formulations are a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). Electrodes are essential parts of batteries that can consist of a variety of materials depending on the type of battery. The electrophore, invented by Johan Wilcke, was an early version of an electrode used to study static electricity. Anode and cathode in electrochemical cells Electrodes are an essential part of any battery. The first electrochemical battery made was devised by Alessandro Volta and was aptly named the Voltaic cell. This battery consisted of a stack of copper and zinc electrodes separated by brine-soaked paper disks. Due to fluctuation in the voltage provided by the voltaic cell it wasn't very practical. The first practical battery was invented in 1839 and named the Daniell cell after John Frederic Daniell. Still making use of the zinc–copper electrode combination. Since then many more batteries ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Suero Oral
In the United States, Suero Oral® is a brand name of an electrolyte solution used to re-hydrate after working in heat-intensive environments, athletic activity, to treat pediatric vomiting and diarrhea, and as a hangover remedy. The product is similar in formula to other popular pediatric electrolyte beverages such as Pedialyte®. The name originated as a reference to suero casero, a whey-based home remedy (also known simply as suero) given to children in parts of South and Central America, the Caribbean, and other Spanish speaking areas. These homemade solutions are common to many households and used to combat dehydration caused by illness, work in extreme heat, or by certain diseases. Oftentimes, in these regions, these homemade solutions are referred to casually as ''suero casero'' (homemade serum), or ''sueros'', but this usage has not extended to the United States. In the United States, the product Suero Oral® contains a blend of water with sugars, flavoring agents (e.g. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sports Drink
Sports drinks, also known as electrolyte drinks, are functional beverages whose stated purpose is to help athletes replace water, electrolytes, and energy before, during and especially after training or competition. There are many perceived benefits and questions pertaining to the efficacy of use for sports and fitness performance. At times if used too much, in wrong cases (as most of nutritional items) they may hinder health or performance. The drinks, or some of their ingredients such as sugar, may not be suitable for certain conditions. Categories of sport drinks Sports drinks can be split into three major types: * Isotonic sport drinks contain similar concentrations of salt and sugar as in the human body. * Hypertonic sport drinks contain a higher concentration of salt and sugar than the human body. * Hypotonic sport drinks contain a lower concentration of salt and sugar than the human body. Most sports drinks are approximately isotonic, having between 4 and 5 heaped teasp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salt (chemistry)
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions. The component ions in a salt compound can be either inorganic, such as chloride (Cl−), or organic, such as acetate (). Each ion can be either monatomic, such as fluoride (F−), or polyatomic, such as sulfate (). Types of salt Salts can be classified in a variety of ways. Salts that produce hydroxide ions when dissolved in water are called ''alkali salts'' and salts that produce hydrogen ions when dissolved in water are called ''acid salts''. ''Neutral salts'' are those salts that are neither acidic nor basic. Zwitterions contain an anionic and a cationic centre in the same molecule, but are not considered salts. Examples of zwitterions are amino acids, many metabolites, peptides, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts such as sodium chloride are often very soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Less frequently, the word ''chloride'' may also form part of the "common" name of chemical compounds in which one or more chlorine atoms are covalently bonded. For example, methyl chloride, with the standard name chloromethane (see IUPAC books) is an organic compound with a covalent C−Cl bond in which the chlorine is not an anion. Electronic properties A chloride ion (diameter 167 pm) is much lar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pedialyte
Pedialyte is an oral electrolyte solution manufactured by Abbott Laboratories and marketed for use in children. It was invented by Dr. Gary Cohen of Swampscott, Massachusetts. Description Pedialyte is designed to promote rehydration and electrolyte replacement in ill children. It "meets the requirements of the American Academy of Pediatrics (AAP) Committee on Nutrition to help prevent dehydration in infants and children." Pedialyte is lower in sugars than most sports drinks, containing 100 calories per liter compared to approximately 240 in Gatorade. It contains more sodium (1,035 milligrams per liter vs. 465 mg/L in Gatorade) and potassium (780 milligrams per liter vs. 127 mg/L in Gatorade). Pedialyte does not contain sucrose, because this sugar has the potential to make diarrhea worse by drawing water into the intestine, increasing the risk of dehydration. In its flavored formulations, Pedialyte uses the synthetic sweeteners sucralose and acesulfame potassium. Pedialyte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vomiting
Vomiting (also known as emesis and throwing up) is the involuntary, forceful expulsion of the contents of one's stomach through the mouth and sometimes the nose. Vomiting can be the result of ailments like food poisoning, gastroenteritis, pregnancy, motion sickness, or hangover; or it can be an after effect of diseases such as brain tumors, elevated intracranial pressure, or overexposure to ionizing radiation. The feeling that one is about to vomit is called nausea; it often precedes, but does not always lead to vomiting. Impairment due to alcohol or anesthesia can cause inhalation of vomit, leading to suffocation. In severe cases, where dehydration develops, intravenous fluid may be required. Antiemetics are sometimes necessary to suppress nausea and vomiting. Self-induced vomiting can be a component of an eating disorder such as bulimia, and is itself now classified as an eating disorder on its own, purging disorder. Complications Aspiration Vomiting is dan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |