|
Standard State
The standard state of a material (pure substance, mixture or solution) is a reference point used to calculate its properties under different conditions. A degree sign (°) or a superscript ⦵ symbol (⦵) is used to designate a thermodynamic quantity in the standard state, such as change in enthalpy (Δ''H''°), change in entropy (Δ''S''°), or change in Gibbs free energy (Δ''G''°). The degree symbol has become widespread, although the Plimsoll is recommended in standards, see discussion about typesetting below. In principle, the choice of standard state is arbitrary, although the International Union of Pure and Applied Chemistry (IUPAC) recommends a conventional set of standard states for general use. The standard state should not be confused with standard temperature and pressure (STP) for gases, nor with the standard solutions used in analytical chemistry. STP is commonly used for calculations involving gases that approximate an ideal gas, whereas standard state condit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Substance
A chemical substance is a unique form of matter with constant chemical composition and characteristic properties. Chemical substances may take the form of a single element or chemical compounds. If two or more chemical substances can be combined without reacting, they may form a chemical mixture. If a mixture is separated to isolate one chemical substance to a desired degree, the resulting substance is said to be chemically pure. Chemical substances can exist in several different physical states or phases (e.g. solids, liquids, gases, or plasma) without changing their chemical composition. Substances transition between these phases of matter in response to changes in temperature or pressure. Some chemical substances can be combined or converted into new substances by means of chemical reactions. Chemicals that do not possess this ability are said to be inert. Pure water is an example of a chemical substance, with a constant composition of two hydrogen atoms bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
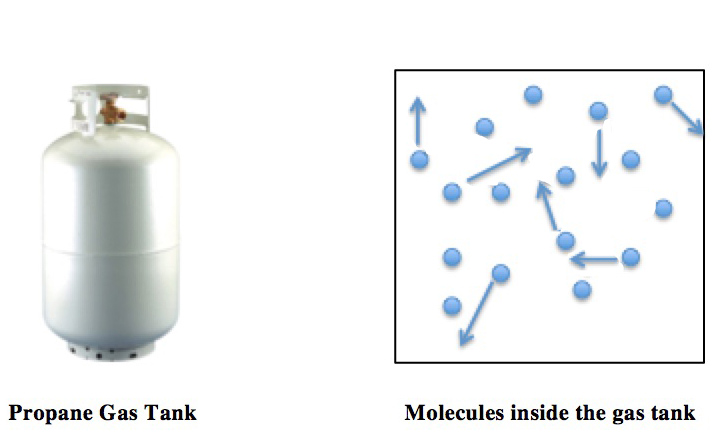
Ideal Gas Equation
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stated by Benoît Paul Émile Clapeyron in 1834 as a combination of the empirical Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law. The ideal gas law is often written in an empirical form: pV = nRT where p, V and T are the pressure, volume and temperature respectively; n is the amount of substance; and R is the ideal gas constant. It can also be derived from the microscopic kinetic theory, as was achieved (independently) by August Krönig in 1856 and Rudolf Clausius in 1857. Equation The state of an amount of gas is determined by its pressure, volume, and temperature. The modern form of the equation relates these simply in two main forms. The temperature used in the equation of state is an absolute temperature: the ap ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Langmuir Adsorption Model
The Langmuir adsorption model explains adsorption by assuming an adsorbate behaves as an ideal gas at isothermal conditions. According to the model, adsorption and desorption are reversible processes. This model even explains the effect of pressure; ''i.e.'', at these conditions the adsorbate's partial pressure p_A is related to its volume adsorbed onto a solid adsorbent. The adsorbent, as indicated in the figure, is assumed to be an ideal solid surface composed of a series of distinct sites capable of binding the adsorbate. The adsorbate binding is treated as a chemical reaction between the adsorbate gaseous molecule A_\text and an empty sorption site . This reaction yields an adsorbed species A_\text with an associated equilibrium constant K_\text: : A_ + S A_. From these basic hypotheses the mathematical formulation of the Langmuir adsorption isotherm can be derived in various independent and complementary ways: by the kinetics, the thermodynamics, and the statistical mech ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activity Coefficients
In thermodynamics, an activity coefficient is a factor used to account for deviation of a mixture of chemical substances from ideal behaviour. In an ideal mixture, the microscopic interactions between each pair of chemical species are the same (or macroscopically equivalent, the enthalpy change of solution and volume variation in mixing is zero) and, as a result, properties of the mixtures can be expressed directly in terms of simple concentrations or partial pressures of the substances present e.g. Raoult's law. Deviations from ideality are accommodated by modifying the concentration by an ''activity coefficient''. Analogously, expressions involving gases can be adjusted for non-ideality by scaling partial pressures by a fugacity coefficient. The concept of activity coefficient is closely linked to that of activity in chemistry. Thermodynamic definition The chemical potential, \mu_\mathrm, of a substance B in an ideal mixture of liquids or an ideal solution is given by :\ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between Electric potential, electrical potential difference and identifiable chemical change. These reactions involve Electron, electrons moving via an electronically conducting phase (typically an external electrical circuit, but not necessarily, as in Electroless nickel-phosphorus plating, electroless plating) between electrodes separated by an ionically conducting and electronically insulating electrolyte (or ionic chemical species, species in a Solution (chemistry), solution). When a chemical reaction is driven by an electrical Voltage, potential difference, as in electrolysis, or if a potential difference results from a chemical reaction as in an electric battery or fuel cell, it is called an ''electrochemical'' reaction. Unlike in other chemical reactions, in electrochemical reactions electrons are not transferred directly between atoms, ions, or molecules, but via the aforementioned electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biochemistry
Biochemistry, or biological chemistry, is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology, and metabolism. Over the last decades of the 20th century, biochemistry has become successful at explaining living processes through these three disciplines. Almost all List of life sciences, areas of the life sciences are being uncovered and developed through biochemical methodology and research.#Voet, Voet (2005), p. 3. Biochemistry focuses on understanding the chemical basis that allows biomolecule, biological molecules to give rise to the processes that occur within living Cell (biology), cells and between cells,#Karp, Karp (2009), p. 2. in turn relating greatly to the understanding of tissue (biology), tissues and organ (anatomy), organs as well as organism structure and function.#Miller, Miller (2012). p. 62. Biochemistry is closely ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amount Concentration
Quantity or amount is a property that can exist as a multitude or magnitude, which illustrate discontinuity and continuity. Quantities can be compared in terms of "more", "less", or "equal", or by assigning a numerical value multiple of a unit of measurement. Mass, time, distance, heat, and angle are among the familiar examples of quantitative properties. Quantity is among the basic classes of things along with quality, substance, change, and relation. Some quantities are such by their inner nature (as number), while others function as states (properties, dimensions, attributes) of things such as heavy and light, long and short, broad and narrow, small and great, or much and little. Under the name of multitude comes what is discontinuous and discrete and divisible ultimately into indivisibles, such as: ''army, fleet, flock, government, company, party, people, mess (military), chorus, crowd'', and ''number''; all which are cases of collective nouns. Under the name of magnit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molality
In chemistry, molality is a measure of the amount of solute in a solution relative to a given mass of solvent. This contrasts with the definition of '' molarity'' which is based on a given volume of solution. A commonly used unit for molality is the moles per kilogram (mol/kg). A solution of concentration 1 mol/kg is also sometimes denoted as 1 molal. The unit mol/kg requires that molar mass be expressed in kg/mol, instead of the usual g/mol or kg/kmol. Definition The molality (''b''), of a solution is defined as the amount of substance (in moles) of solute, ''n''solute, divided by the mass (in kg) of the solvent, ''m''solvent: :b = \frac. In the case of solutions with more than one solvent, molality can be defined for the mixed solvent considered as a pure pseudo-solvent. Instead of mole solute per kilogram solvent as in the binary case, units are defined as mole solute per kilogram mixed solvent. Origin The term ''molality'' is formed in analogy to ''molari ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metastability
In chemistry and physics, metastability is an intermediate energetic state within a dynamical system other than the system's state of least energy. A ball resting in a hollow on a slope is a simple example of metastability. If the ball is only slightly pushed, it will settle back into its hollow, but a stronger push may start the ball rolling down the slope. Bowling pins show similar metastability by either merely wobbling for a moment or tipping over completely. A common example of metastability in science is isomerisation. Higher energy isomers are long lived because they are prevented from rearranging to their preferred ground state by (possibly large) barriers in the potential energy. During a metastable state of finite lifetime, all state-describing parameters reach and hold stationary values. In isolation: *the state of least energy is the only one the system will inhabit for an indefinite length of time, until more external energy is added to the system (unique "absolu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared artificially, the two most common allotropes being white phosphorus and red phosphorus. With as its only stable isotope, phosphorus has an occurrence in Earth's crust of about 0.1%, generally as phosphate rock. A member of the pnictogen family, phosphorus readily forms a wide variety of organic compound, organic and inorganic compound, inorganic compounds, with as its main oxidation states +5, +3 and −3. The isolation of white phosphorus in 1669 by Hennig Brand marked the scientific community's first discovery since Antiquity of an element. The name phosphorus is a reference to the Phosphorus (morning star), god of the Morning star in Greek mythology, inspired by the faint glow of white phosphorus when exposed to oxygen. This property is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
White Tin
White tin is refined metallic tin. It contrasts with black tin, which is unrefined tin ore (cassiterite) as extracted from the ground. The term "white tin" was historically associated with tin mining in Devon and Cornwall where it was smelted from black tin in blowing houses. White tin may also refer specifically to β-tin, the metallic allotrope of the pure element, as opposed to the nonmetallic allotrope α-tin (also known as gray tin), which occurs at temperatures below , a transformation known as tin pest). White tin has tetragonal unit cell. References * * * * White White is the lightest color and is achromatic (having no chroma). It is the color of objects such as snow, chalk, and milk, and is the opposite of black. White objects fully (or almost fully) reflect and scatter all the visible wa ... Mining in Cornwall {{mining-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, carbon-12, C and carbon-13, C being stable, while carbon-14, C is a radionuclide, decaying with a half-life of 5,700 years. Carbon is one of the timeline of chemical element discoveries#Pre-modern and early modern discoveries, few elements known since antiquity. Carbon is the 15th abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the abundance of the chemical elements, fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual abi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |