|
Salivation
Saliva (commonly referred as spit or drool) is an extracellular fluid produced and secreted by salivary glands in the mouth. In humans, saliva is around 99% water, plus electrolytes, mucus, white blood cells, epithelial cells (from which DNA can be extracted), enzymes (such as lingual lipase and amylase), and antimicrobial agents (such as secretory IgA, and lysozymes). The enzymes found in saliva are essential in beginning the process of digestion of dietary starches and fats. These enzymes also play a role in breaking down food particles entrapped within dental crevices, thus protecting teeth from bacterial decay. Saliva also performs a lubricating function, wetting food and permitting the initiation of swallowing, and protecting the oral mucosa from drying out. Saliva has specialized purposes for a variety of animal species beyond predigestion. Certain swifts construct nests with their sticky saliva. The foundation of bird's nest soup is an aerodramus nest. Venomous sa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salivary Gland
The salivary glands in many vertebrates including mammals are exocrine glands that produce saliva through a system of ducts. Humans have three paired major salivary glands ( parotid, submandibular, and sublingual), as well as hundreds of minor salivary glands. Salivary glands can be classified as serous, mucous, or seromucous (mixed). In serous secretions, the main type of protein secreted is alpha-amylase, an enzyme that breaks down starch into maltose and glucose, whereas in mucous secretions, the main protein secreted is mucin, which acts as a lubricant. In humans, 1200 to 1500 ml of saliva are produced every day. The secretion of saliva (salivation) is mediated by parasympathetic stimulation; acetylcholine is the active neurotransmitter and binds to muscarinic receptors in the glands, leading to increased salivation. A proposed fourth pair of salivary glands, the tubarial glands, were first identified in 2020. They are named for their location, being positione ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
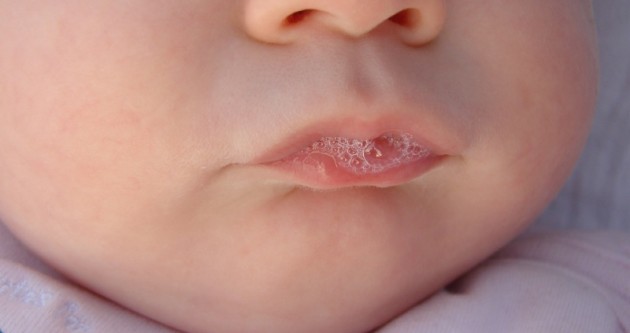
Saliva Baby
Saliva (commonly referred as spit or drool) is an extracellular fluid produced and secreted by salivary glands in the mouth. In humans, saliva is around 99% water, plus electrolytes, mucus, white blood cells, epithelial cells (from which DNA can be extracted), enzymes (such as lingual lipase and amylase), and antimicrobial agents (such as secretory IgA, and lysozymes). The enzymes found in saliva are essential in beginning the process of digestion of dietary starches and fats. These enzymes also play a role in breaking down food particles entrapped within dental crevices, thus protecting teeth from bacterial decay. Saliva also performs a lubricating function, wetting food and permitting the initiation of swallowing, and protecting the oral mucosa from drying out. Saliva has specialized purposes for a variety of animal species beyond predigestion. Certain swifts construct nests with their sticky saliva. The foundation of bird's nest soup is an aerodramus nest. Venomous ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food
Food is any substance consumed by an organism for Nutrient, nutritional support. Food is usually of plant, animal, or Fungus, fungal origin and contains essential nutrients such as carbohydrates, fats, protein (nutrient), proteins, vitamins, or Mineral (nutrient), minerals. The substance is Ingestion, ingested by an organism and assimilated by the organism's Cell (biology), cells to provide energy, maintain life, or stimulate growth. Different species of animals have different List of feeding behaviours, feeding behaviours that satisfy the needs of their metabolisms and have evolved to fill a specific ecological niche within specific geographical contexts. Omnivore, Omnivorous humans are highly adaptable and have adapted to obtaining food in many different ecosystems. Humans generally use cooking to prepare food for consumption. The majority of the food energy required is supplied by the industrial food industry, which produces food through Intensive farming, intensive agricu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pronunciation of the word "chloride" is . Chloride salts such as sodium chloride are often soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Other examples of ionic chlorides include potassium chloride (), calcium chloride (), and ammonium chloride (). Examples of covalent chlorides include methyl chloride (), carbon tetrachloride (), sulfuryl chloride (), and monochloramine (). Electronic properties A chloride ion (diameter 167 pm) is much larger than a chlorine atom (diameter 99 pm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table), it occurs naturally only in combination with other elements and almost always has an oxidation state of +2. It reacts readily with air to form a thin Passivation (chemistry), passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium Salt (chemistry), salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight magnesium alloy, alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three Helium nucleus, helium nuclei to a carbon nucleus. When such stars explo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossils of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name comes from Latin ''calx'' " lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and pharmaceuticals for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium
Potassium is a chemical element; it has Symbol (chemistry), symbol K (from Neo-Latin ) and atomic number19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, which is easily removed to create cation, an ion with a positive charge (which combines with anions to form salts). In nature, potassium occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac-flame color, colored flame. It is found dissolved in seawater (which is 0.04% potassium by weight), and occurs in many minerals such as orthoclase, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood Plasma
Blood plasma is a light Amber (color), amber-colored liquid component of blood in which blood cells are absent, but which contains Blood protein, proteins and other constituents of whole blood in Suspension (chemistry), suspension. It makes up about 55% of the body's total blood volume. It is the Intravascular compartment, intravascular part of extracellular fluid (all body fluid outside cells). It is mostly water (up to 95% by volume), and contains important dissolved proteins (6–8%; e.g., serum albumins, globulins, and fibrinogen), glucose, clotting factors, electrolytes (, , , , , etc.), hormones, carbon dioxide (plasma being the main medium for excretory product transportation), and oxygen. It plays a vital role in an intravascular osmotic effect that keeps electrolyte concentration balanced and protects the body from infection and other blood-related disorders. Blood plasma can be separated from whole blood through blood fractionation, by adding an anticoagulant to a tube ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature and must be prepared from compounds. Sodium is the Abundance of elements in Earth's crust, sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been Leaching (chemistry), leached by the action of water from the Earth, Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide (lye) is used in Soap, soap manufac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolyte
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons. This includes most soluble Salt (chemistry), salts, acids, and Base (chemistry), bases, dissolved in a polar solvent like water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved. Electrically, such a solution is neutral. If an electric potential is applied to such a solution, the cations of the solution are drawn to the electrode that has an abundance of electrons, while the anions are drawn to the electrode that has a deficit of electrons. The movement of anions and cations in opposite directions within the solution amounts to a current. Some gases, such as hydrogen chloride (HCl), under conditions of high temperature or low pressure can also functi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecules known as product (chemistry), products. Almost all metabolism, metabolic processes in the cell (biology), cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme, pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts include Ribozyme, catalytic RNA molecules, also called ribozymes. They are sometimes descr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolyte
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons. This includes most soluble Salt (chemistry), salts, acids, and Base (chemistry), bases, dissolved in a polar solvent like water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved. Electrically, such a solution is neutral. If an electric potential is applied to such a solution, the cations of the solution are drawn to the electrode that has an abundance of electrons, while the anions are drawn to the electrode that has a deficit of electrons. The movement of anions and cations in opposite directions within the solution amounts to a current. Some gases, such as hydrogen chloride (HCl), under conditions of high temperature or low pressure can also functi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |