|
Ryanodine
Ryanodine is a poisonous diterpenoid found in the South American plant ''Ryania speciosa'' (Salicaceae). It was originally used as an insecticide. The compound has extremely high affinity to the open-form ryanodine receptor, a group of calcium channels found in skeletal muscle, smooth muscle, and heart muscle cells. It binds with such high affinity to the receptor that it was used as a label for the first purification of that class of ion channels and gave its name to it. At nanomolar concentrations, ryanodine locks the receptor in a half-open state, whereas it fully closes them at micromolar concentration. The effect of the nanomolar-level binding is that ryanodine causes release of calcium from calcium stores as the sarcoplasmic reticulum in the cytoplasm, leading to massive muscle contractions. The effect of micromolar-level binding is paralysis. This is true for both mammals and insects. See also * Ryanoid Ryanoids are a class of insecticides which share the same mechanism ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ryanodine Receptor
Ryanodine receptors (RyR for short) form a class of intracellular calcium channels in various forms of excitable animal tissue like muscles and neurons. There are three major isoforms of the ryanodine receptor, which are found in different tissues and participate in different signaling pathways involving calcium release from intracellular organelles. The RYR2 ryanodine receptor isoform is the major cellular mediator of calcium-induced calcium release (CICR) in animal cells. Etymology The ryanodine receptors are named after the plant alkaloid ryanodine which shows a high affinity to them. Isoforms There are multiple isoforms of ryanodine receptors: * RyR1 is primarily expressed in skeletal muscle * RyR2 is primarily expressed in myocardium (heart muscle) * RyR3 is expressed more widely, but especially in the brain. * Non-mammalian vertebrates typically express two RyR isoforms, referred to as RyR-alpha and RyR-beta. * Many invertebrates, including the model organisms Dros ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sarcoplasmic Reticulum
The sarcoplasmic reticulum (SR) is a membrane-bound structure found within muscle cells that is similar to the smooth endoplasmic reticulum in other Cell (biology), cells. The main function of the SR is to store calcium ions (Ca2+). Calcium in biology, Calcium ion levels are kept relatively constant, with the concentration of calcium ions within a cell being 10,000 times smaller than the concentration of calcium ions outside the cell. This means that small increases in calcium ions within the cell are easily detected and can bring about important cellular changes (the calcium is said to be a second messenger). Calcium is used to make calcium carbonate (found in chalk) and calcium phosphate, two compounds that the body uses to make teeth and bones. This means that too much calcium within the cells can lead to hardening (calcification) of certain intracellular structures, including the mitochondrion, mitochondria, leading to cell death. Therefore, it is vital that calcium ion levels a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Insecticide
Insecticides are substances used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. Insecticides are used in agriculture, medicine, industry and by consumers. Insecticides are claimed to be a major factor behind the increase in the 20th-century's agricultural productivity. Nearly all insecticides have the potential to significantly alter ecosystems; many are toxic to humans and/or animals; some become concentrated as they spread along the food chain. Insecticides can be classified into two major groups: systemic insecticides, which have residual or long term activity; and contact insecticides, which have no residual activity. The mode of action describes how the pesticide kills or inactivates a pest. It provides another way of classifying insecticides. Mode of action can be important in understanding whether an insecticide will be toxic to unrelated species, such as fish, birds and mammals. Insecticides may be repellent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
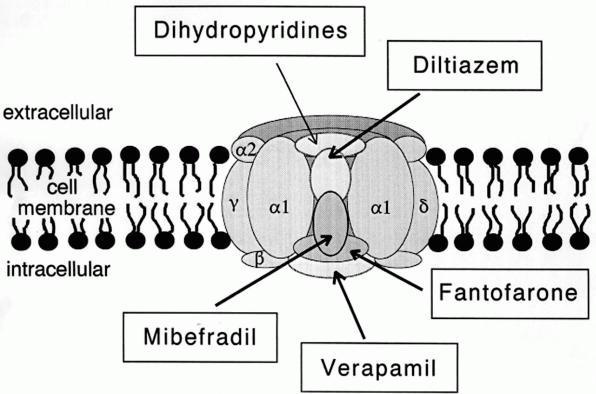
Calcium Channel
A calcium channel is an ion channel which shows selective permeability to calcium ions. It is sometimes synonymous with voltage-gated calcium channel, although there are also ligand-gated calcium channels. Comparison tables The following tables explain gating, gene, location and function of different types of calcium channels, both voltage and ligand-gated. Voltage-gated Ligand-gated *the ''receptor-operated calcium channels'' (in vasoconstriction) **P2X receptors Page 479 Pharmacology L-type calcium channel blockers are used to treat hypertension. In most areas of the body, depolarization is mediated by sodium influx into a cell; changing the calcium permeability has little effect on action potentials. However, in many smooth muscle tissues, depolarization is mediated primarily by calcium influx into the cell. L-type calcium channel blockers selectively inhibit these action potentials in smooth muscle which leads to dilation of blood vessels; this in turn corrects hypert ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Insecticides
Insecticides are substances used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. Insecticides are used in agriculture, medicine, industry and by consumers. Insecticides are claimed to be a major factor behind the increase in the 20th-century's agricultural productivity. Nearly all insecticides have the potential to significantly alter ecosystems; many are toxic to humans and/or animals; some become concentrated as they spread along the food chain. Insecticides can be classified into two major groups: systemic insecticides, which have residual or long term activity; and contact insecticides, which have no residual activity. The mode of action describes how the pesticide kills or inactivates a pest. It provides another way of classifying insecticides. Mode of action can be important in understanding whether an insecticide will be toxic to unrelated species, such as fish, birds and mammals. Insecticides may be repellent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ryanoid
Ryanoids are a class of insecticides which share the same mechanism of action as the alkaloid ryanodine. Ryanodine is a naturally occurring insecticide isolated from ''Ryania speciosa''. Ryanoids include natural chemicals which are closely related to ryanodine, such as ryanodol and 9,21-didehydroryanodol, and also chemically distinct synthetic compounds such as chlorantraniliprole (Rynaxypyr), flubendiamide, cyantraniliprole, cyclaniliprole, and tetraniliprole, which are called diamide insecticides. Ryanoids exert their insecticidal effect by interacting with ryanodine receptors, a type of calcium channel A calcium channel is an ion channel which shows selective permeability to calcium ions. It is sometimes synonymous with voltage-gated calcium channel, although there are also ligand-gated calcium channels. Comparison tables The following tables e .... This causes loss of muscle function leading to paralysis and death. References {{insecticides Insecticides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paralysis
Paralysis (also known as plegia) is a loss of motor function in one or more muscles. Paralysis can also be accompanied by a loss of feeling (sensory loss) in the affected area if there is sensory damage. In the United States, roughly 1 in 50 people have been diagnosed with some form of permanent or transient paralysis. The word "paralysis" derives from the Greek παράλυσις, meaning "disabling of the nerves" from παρά (''para'') meaning "beside, by" and λύσις (''lysis'') meaning "making loose". A paralysis accompanied by involuntary tremors is usually called "palsy". Causes Paralysis is most often caused by damage in the nervous system, especially the spinal cord. Other major causes are stroke, trauma with nerve injury, poliomyelitis, cerebral palsy, peripheral neuropathy, Parkinson's disease, ALS, botulism, spina bifida, multiple sclerosis, and Guillain–Barré syndrome. Temporary paralysis occurs during REM sleep, and dysregulation of this system can lead ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diterpene Alkaloids
Diterpenes are a class of chemical compounds composed of four isoprene units, often with the molecular formula C20H32. They are biosynthesized by plants, animals and fungi via the HMG-CoA reductase pathway, with geranylgeranyl pyrophosphate being a primary intermediate. Diterpenes form the basis for biologically important compounds such as retinol, retinal, and phytol. They are known to be antimicrobial and antiinflammatory. Structures As with most terpenes a huge number of potential structures exists, which may be broadly divided according to the number of rings present. Biosynthesis Diterpenes are derived from the addition of one IPP unit to FPP to form geranylgeranyl-pyrophosphate (GGPP). From GGPP, structural diversity is achieved mainly by two classes of enzymes; the diterpene synthases and cytochromes P450. Several diterpenes are produced by plants and cyanobacteria. GGPP is also the precursor for the synthesis of the phytane by the action of the enzyme geranylger ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentanes
Cyclopentane (also called C pentane) is a highly flammable alicyclic hydrocarbon with chemical formula C5H10 and CAS number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and below the plane. It occurs as a colorless liquid with a petrol-like odor. Its melting point is −94 °C and its boiling point is 49 °C. Cyclopentane is in the class of cycloalkanes, being alkanes that have one or more rings of carbon atoms. It is formed by cracking cyclohexane in the presence of alumina at a high temperature and pressure. It was first prepared in 1893 by the German chemist Johannes Wislicenus. Production, occurrence and use Cycloalkanes are formed by catalytic reforming. For example, when passed over a hot platinum surfact, 2-methylbutane converts into cyclopentane. Cyclopentane has no particular use. No commercial products are made from cyclopentane itself. As a volatile hydrocarbon it is an incidental component of some fuels and blowing a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohols
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest examples, includes all compounds which conform to the general formula . Simple monoalcohols that are the subject of this article include primary (), secondary () and tertiary () alcohols. The suffix ''-ol'' appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority. When a higher priority group is present in the compound, the prefix ''hydroxy-'' is used in its IUPAC name. The suffix ''-ol'' in non-IUPAC names (such as paracetamol or cholesterol) also typically indicates that the substance is an alcohol. However, some compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylate Esters
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, (or ). It is an ion with negative charge. Carboxylate salts are salts that have the general formula , where M is a metal and ''n'' is 1, 2,...; ''carboxylate esters'' have the general formula (or ). R and R′ are organic groups; R′ ≠ H. Synthesis Carboxylate ions can be formed by deprotonation of carboxylic acids. Such acids typically have p''K''a of less than 5, meaning that they can be deprotonated by many bases, such as sodium hydroxide or sodium bicarbonate. :RCOOH + NaOH -> RCOONa + H2O Resonance stabilization of the carboxylate ion Carboxylic acids easily dissociate into a carboxylate anion and a positively charged hydrogen ion (proton), much more readily than alcohols do (into an alkoxide ion and a proton), because the carboxylate ion is stabilized by resonance. The negative charge that is left after deprotonation of the carboxyl group is delocalized between the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrroles
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme. Pyrroles are components of more complex macrocycles, including the porphyrinogens and products derived therefrom, including porphyrins of heme, the chlorins, bacteriochlorins, and chlorophylls. Properties Pyrrole is a colorless volatile liquid that darkens readily upon exposure to air, and is usually purified by distillation immediately before use. Pyrrole has a nutty odor. Pyrrole is a 5-membered aromatic heterocycle, like furan and thiophene. Unlike furan and thiophene, it has a dipole in which the positive end lies on the side of the heteroatom, with a dipole moment of 1.58 D. In CDCl3, it has chemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |