Carboxylate Esters on:
[Wikipedia]
[Google]
[Amazon]

 In
In
RCOOH + NaOH -> RCOONa + H2O
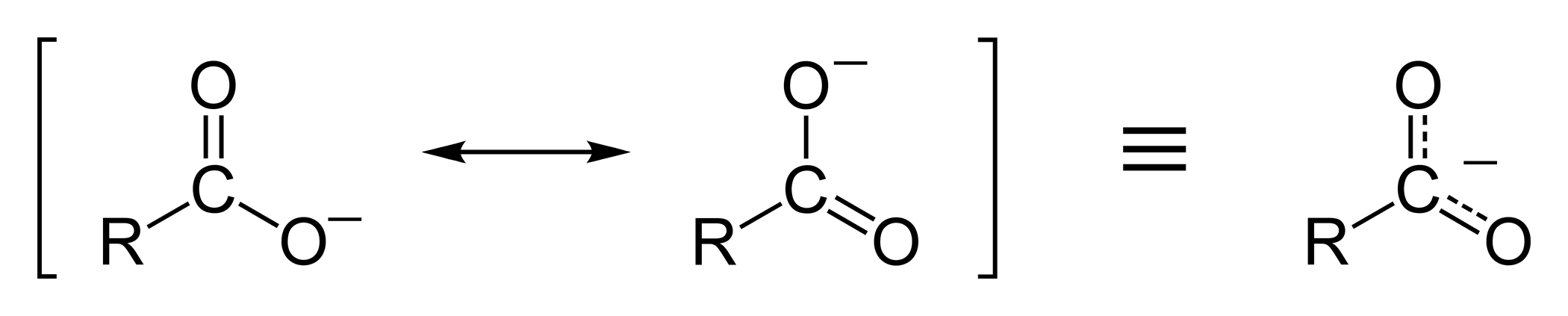 This
This
 The nucleophilicity of carboxylate ions are much weaker than that of
The nucleophilicity of carboxylate ions are much weaker than that of



 In
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
, a carboxylate is the conjugate base
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid donates a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as in the reverse reaction it loses a ...
of a carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
, (or ). It is an ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
with negative charge
Electric charge is the physical property of matter that causes charged matter to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative'' (commonly carried by protons and electrons respectiv ...
.
Carboxylate salts are salts
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively cha ...
that have the general formula , where M is a metal and ''n'' is 1, 2,...; ''carboxylate esters'' have the general formula (or ). R and R′ are organic groups; R′ ≠ H.
Synthesis
Carboxylate ions can be formed by deprotonation of carboxylic acids. Such acids typically have p''K''a of less than 5, meaning that they can be deprotonated by many bases, such assodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkali ...
or sodium bicarbonate
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation ( Na+) and a bicarbonate anion ( HCO3−) ...
.
:Resonance stabilization of the carboxylate ion
Carboxylic acids easily dissociate into a carboxylate anion and a positively charged hydrogen ion (proton), much more readily than alcohols do (into analkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organic substituent. Alkoxides are strong bases and, whe ...
ion and a proton), because the carboxylate ion is stabilized by resonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillatin ...
. The negative charge that is left after deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.ed ...
of the carboxyl group is delocalized between the two electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
oxygen atoms in a resonance structure. If the R group is an electron-withdrawing group (such as –CF3), the basicity of the carboxylate will be further weakened.
: This
This delocalization
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly dif ...
of the electron cloud means that both of the oxygen atoms are less strongly negatively charged; the positive proton is therefore less strongly attracted back to the carboxylate group once it has left; hence, the carboxylate ion is more stable and less basic as a result of resonance stabilization
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or '' ...
of the negative charge. In contrast, an alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organic substituent. Alkoxides are strong bases and, whe ...
ion, once formed, would have a strong negative charge localized on its lone oxygen atom, which would strongly attract any nearby protons (indeed, alkoxides are very strong bases). Because of resonance stabilization, carboxylic acids have much lower p''K''a values (and are therefore stronger acids) than alcohols
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is ...
. For example, the p''K''a value of acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component ...
is 4.8, while ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl ...
has a p''K''a of 16. Hence acetic acid is a much stronger acid than ethanol. This in turn means that for equimolar solutions of a carboxylic acid and an alcohol, the carboxylic acid would have a much lower pH.
Reactions
Nucleophilic substitution
Carboxylate ions are goodnucleophiles
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
. They react with alkyl halides
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely us ...
to form ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
. The following reaction shows the reaction mechanism.
 The nucleophilicity of carboxylate ions are much weaker than that of
The nucleophilicity of carboxylate ions are much weaker than that of hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. I ...
and alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organic substituent. Alkoxides are strong bases and, whe ...
ions, but stronger than halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
anions (in a polar aprotic solvent A polar aprotic solvent is a solvent that lacks an acidic proton and is polar. Such solvents lack hydroxyl and amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Am ...
, though there are other effects such as solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubil ...
of the ion).
Reduction
Unlike the reduction of ester, the reduction of carboxylate is different, due to the lack of theleaving group In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited t ...
and the relatively electron-rich carbon atom (due to the negative charge on the oxygen atoms). With a small amount of acid, the reaction occurs with lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li Al H4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic ...
by changing the LAH into the Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
AlH3 in the process, converting the oxyanion to 4 Al–O bonds.
Examples
This list is for cases where there is a separate article for the anion or its derivatives. All other organic acids should be found at their parent carboxylic acid. * Formate ion, HCOO− *Acetate
An acetate is a salt (chemistry), salt formed by the combination of acetic acid with a base (e.g. Alkali metal, alkaline, Alkaline earth metal, earthy, Transition metal, metallic, nonmetallic or radical Radical (chemistry), base). "Acetate" als ...
ion, CH3COO−
* Methanetetracarboxylate
In chemistry, methanetetracarboxylate is a tetravalent anion with formula or C(COO−)4. It has four carboxylate groups attached to a central carbon atom; so it has the same carbon backbone as neopentane. It is an oxocarbon anion, that is, co ...
ion, C(COO−)4
* Oxalate
Oxalate (IUPAC: ethanedioate) is an anion with the formula C2O42−. This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (Na2C2O4), and several esters such as dimethyl o ...
ion,
See also
*Carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
References
{{Reflist Anions...........