|
Pfitzner–Moffatt Oxidation
The Pfitzner–Moffatt oxidation, sometimes referred to as simply the Moffatt oxidation, is a chemical reaction for the oxidation of primary and secondary alcohols to aldehydes and ketones, respectively. The oxidant is a combination of dimethyl sulfoxide (DMSO) and dicyclohexylcarbodiimide (DCC). The reaction was first reported by J. Moffatt and his student K. Pfitzner in 1963. Stoichiometry and mechanism The reaction requires one equivalent each of the diimide, which is the dehydrating agent, and the sulfoxide, the oxidant: :(CH3)2SO + (CyN)2C + R2CHOH → (CH3)2S + (CyNH)2CO + R2C=O Typically the sulfoxide and diimide are used in excess. The reaction cogenerates dimethyl sulfide and a urea. Dicyclohexylurea ((CyNH)2CO) can be difficult to remove from the product. In terms of mechanism, the reaction is proposed to involve the intermediary of an sulfonium group, formed by a reaction between DSMO and the carbodiimide. : This species is highly reactive and is attacked by the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (chemistry), products, which usually have properties different from the reactants. Reactions often consist of a sequence o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
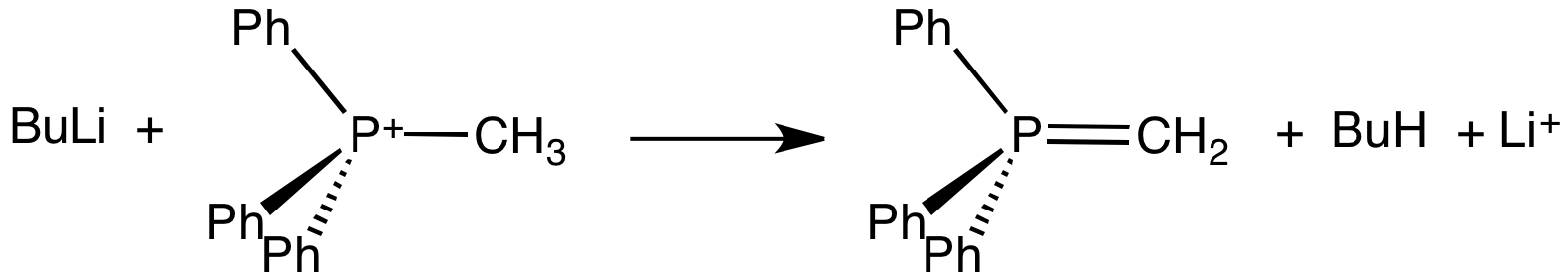
Ylide
An ylide or ylid () is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms have full octets of electrons. The result can be viewed as a structure in which two adjacent atoms are connected by both a covalent and an ionic bond; normally written X+–Y−. Ylides are thus 1,2-dipolar compounds, and a subclass of zwitterions. They appear in organic chemistry as reagents or reactive intermediates. The class name "ylide" for the compound should not be confused with the suffix "-ylide". Resonance structures Many ylides may be depicted by a multiple bond form in a resonance structure, known as the ylene form, while the actual structure lies in between both forms: : The actual bonding picture of these types of ylides is strictly zwitterionic (the structure on the right) with the strong Coulombic attraction between the " ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonium-based Oxidation Of Alcohols To Aldehydes
Sulfonium-based oxidations of alcohols to aldehydes summarizes a group of organic reactions that transform a primary alcohol to the corresponding aldehyde (and a secondary alcohol to the corresponding ketone). Selective redox, oxidation of alcohols to aldehydes requires circumventing Oxidation of primary alcohols to carboxylic acids, over-oxidation to the carboxylic acid. One popular approach are methods that proceed through intermediate alkoxysulfonium species (, e.g. compound 6) as detailed here. Since most of these methods employ dimethylsulfoxide (DMSO) as oxidant and generate dimethylsulfide, these are often colloquially summarized as DMSO-oxidations. Conceptually, generating an aldehyde and dimethylsulfide from an alcohol and DMSO requires a dehydrating agent for removal of H2O, ideally an electrophile simultaneously activating DMSO. In contrast, methods generating the sulfonium intermediate from dimethylsulfide do not require a dehydrating agent. Closely related are oxidations ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol Oxidation
Alcohol oxidation is a class of organic reactions in which the alcohol functional group is converted into another functional group (e.g., aldehyde, ketone, carboxylic acid) in which carbon carries a higher oxidation state. Through a variety of mechanisms, the removal of a hydride equivalent converts a primary or secondary alcohol to an aldehyde or ketone, respectively. The oxidation of primary alcohols to carboxylic acids normally proceeds via the corresponding aldehyde, which is transformed via an aldehyde hydrate (''gem''-diol, R-CH(OH)2) by reaction with water. Thus, the oxidation of a primary alcohol at the aldehyde level without further oxidation to the carboxylic acid is possible by performing the reaction in absence of water, so that no aldehyde hydrate can be formed. Oxidation to aldehydes Oxidation of alcohols to aldehydes is partial oxidation; aldehydes are further oxidized to carboxylic acids. Conditions required for making aldehydes are heat and distillation. In aldeh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corey–Kim Oxidation
The Corey–Kim oxidation is an oxidation reaction used to synthesise aldehydes and ketones from primary and secondary alcohols. It is named for American chemist and Nobel Laureate Elias James Corey and Korean-American chemist Choung Un Kim. Although the Corey–Kim oxidation possesses the distinctive advantage over Swern oxidation of allowing an operation above –25 °C, it is not so commonly used due to issues with selectivity in substrates susceptible to chlorination by ''N''-chlorosuccinimide. Reaction mechanism Dimethyl sulfide (Me2S) is treated with ''N''-chlorosuccinimide (NCS), resulting in formation of an "active DMSO" species that is used for the activation of the alcohol. Addition of triethylamine to the activated alcohol leads to its oxidation to aldehyde or ketone and generation of dimethyl sulfide. In variance with other alcohol oxidation using "activated DMSO," the reactive oxidizing species is not generated by reaction of DMSO with an electrophil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parikh–Doering Oxidation
The Parikh– Doering oxidation is an oxidation reaction that transforms primary and secondary alcohols into aldehydes and ketones, respectively. The procedure uses dimethyl sulfoxide (DMSO) as the oxidant and the solvent, activated by the sulfur trioxide pyridine complex (SO3•C5H5N) in the presence of triethylamine or diisopropylethylamine as base. Dichloromethane is frequently used as a cosolvent for the reaction. Compared to other activated DMSO oxidations, the Parikh–Doering oxidation is operationally simple: the reaction can be run at non-cryogenic temperatures, often between 0 °C and room temperature, without formation of significant amounts of methyl thiomethylether side products. However, the Parikh–Doering oxidation sometimes requires a large excess of DMSO, SO3•C5H5N and/or base as well as prolonged reaction times for high conversions and yields to be obtained. The following example from the total synthesis of (–)-kumausallene by P.A. Evans and cowo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Swern Oxidation
The Swern oxidation, named after Daniel Swern, is a chemical reaction whereby a primary or secondary alcohol is oxidized to an aldehyde or ketone using oxalyl chloride, dimethyl sulfoxide (DMSO) and an organic base, such as triethylamine. It is one of the many oxidation reactions commonly referred to as 'activated DMSO' oxidations. The reaction is known for its mild character and wide tolerance of functional groups. The by-products are dimethyl sulfide ((CH3)2S), carbon monoxide (CO), carbon dioxide (CO2) and—when triethylamine is used as base— triethylammonium chloride (Et3NHCl). Of the volatile by-products, dimethyl sulfide has a strong, pervasive odour and carbon monoxide is acutely toxic, so the reaction and the work-up needs to be performed in a fume hood. Dimethyl sulfide is a volatile liquid (B.P. 37 °C) with an unpleasant odour at even low concentrations. Mechanism The first step of the Swern oxidation is the low-temperature reaction of DMSO, 1a, formally as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound. The term carbonyl can also refer to carbon monoxide as a ligand in an inorganic or organometallic complex (a metal carbonyl, e.g. nickel carbonyl). The remainder of this article concerns itself with the organic chemistry definition of carbonyl, where carbon and oxygen share a double bond. Carbonyl compounds In organic chemistry, a carbonyl group characterizes the following types of compounds: Other organic carbonyls are urea and the carbamates, the derivatives of acyl chlorides chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, hydroxamates, and isocyanates. Examples of inorganic carbonyl compounds are carbon dioxide and carbonyl sulfide. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonium
In organic chemistry, a sulfonium ion, also known as sulphonium ion or sulfanium ion, is a positively-charged ion (a " cation") featuring three organic substituents attached to sulfur. These organosulfur compounds have the formula . Together with a negatively-charged counterion, they give sulfonium salts. They are typically colorless solids that are soluble in organic solvent. Synthesis Sulfonium compounds are usually synthesized by the reaction of thioethers with alkyl halides. For example, the reaction of dimethyl sulfide with iodomethane yields trimethylsulfonium iodide: : + → The reaction proceeds by a nucleophilic substitution mechanism (SN2). Iodide is the leaving group departs. The rate of methylation is faster with more electrophilic methylating agents, such as methyl trifluoromethanesulfonate. Inversion Sulfonium ions with three different substituents are chiral owing to their pyramidal structure. Unlike the isoelectronic oxonium ions (R3O+), chiral sulfon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




3S _in_the_BPh4-_salt_(code_HEYZAM).png)