Sulfonium-based Oxidation Of Alcohols To Aldehydes on:
[Wikipedia]
[Google]
[Amazon]
Sulfonium-based oxidations of alcohols to aldehydes summarizes a group of
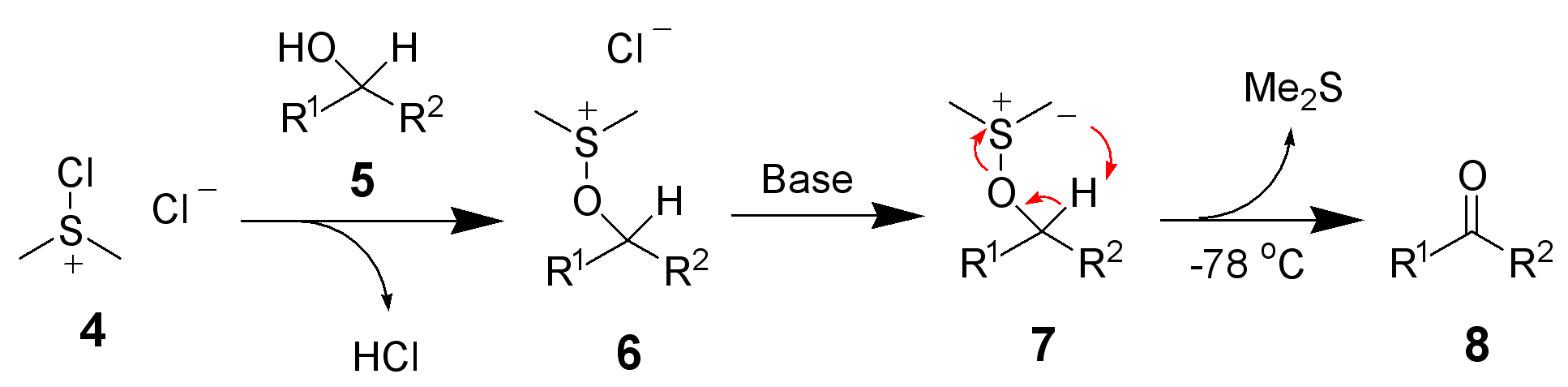 These activated sulfoxides react as electrophiles when treated with an alcohol, expelling a leaving group that might simultaneously function as counter-ion to the alkoxysulfonium species () generated. Upon deprotonation – usually assisted by a mild base like triethylamine – the alkoxysulfonium species decomposes, yielding the aldehyde and dimethylsulfide. The latter collection contains popular oxidations like
* Swern,
* Corey-Kim,
* Parikh-Doering,
* Pfitzner-Moffatt
and also includes Albright-Goldman, Albright-Onodera (DMSO/P2O5), TFAA/DMSO (Swern) and Me2S/Cl2. Recently, SO2F2 has been proposed for generating the activated sulfoxide from DMSO.
These activated sulfoxides react as electrophiles when treated with an alcohol, expelling a leaving group that might simultaneously function as counter-ion to the alkoxysulfonium species () generated. Upon deprotonation – usually assisted by a mild base like triethylamine – the alkoxysulfonium species decomposes, yielding the aldehyde and dimethylsulfide. The latter collection contains popular oxidations like
* Swern,
* Corey-Kim,
* Parikh-Doering,
* Pfitzner-Moffatt
and also includes Albright-Goldman, Albright-Onodera (DMSO/P2O5), TFAA/DMSO (Swern) and Me2S/Cl2. Recently, SO2F2 has been proposed for generating the activated sulfoxide from DMSO.
organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, Mechanistic Organ ...
s that transform a primary alcohol to the corresponding aldehyde (and a secondary alcohol to the corresponding ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
). Selective oxidation of alcohols to aldehydes requires circumventing over-oxidation to the carboxylic acid. One popular approach are methods that proceed through intermediate alkoxysulfonium species (, e.g. compound 6) as detailed here. Since most of these methods employ dimethylsulfoxide (DMSO) as oxidant and generate dimethylsulfide, these are often colloquially summarized as DMSO-oxidations. Conceptually, generating an aldehyde and dimethylsulfide from an alcohol and DMSO requires a dehydrating agent for removal of H2O, ideally an electrophile simultaneously activating DMSO. In contrast, methods generating the sulfonium intermediate from dimethylsulfide do not require a dehydrating agent. Closely related are oxidations mediated by dimethyl selenoxide and by dimethyl selenide.
Comparison with related methods
In comparisons, sulfonium-based methods are popular because reactions are efficient (high yields, comparably fast, no over-oxidation, few side reactions, reproducible results), reaction conditions are mild (low temperature, no strong acids or bases), reactions are operationally simple (no specialized equipment or uncommon and/or costly reagents necessary, byproducts often easily separated, tolerant of oxygen and moisture,) and they generally avoid highly toxic starting materials and toxic waste disposal. However, the reactions are not too popular with many undergraduate chemistry students in the laboratory since the common byproduct dimethylsulfide is a strong odorant, reminiscent of fouling eggs, that requires a well-ventilated fume hood. Other drawbacks might include excess of base, handling of the dehydrating agent, limited choice of solvent or side reactions at elevated temperature, e.g. Pummerer rearrangement or elimination of the sulfonium intermediate to the reactive H2C=(S+)-CH3-species that formmethylthiomethyl ether
In organic chemistry a methylthiomethyl (MTM) ether is a protective group for hydroxyl groups. Hydroxyl groups are present in many chemical compounds and they must be protected during oxidation, acylation, halogenation, dehydration and other reacti ...
s with alcohols. In consequence this means that the activity of the oxidation can not be tuned at will by increasing the reaction temperature, e.g. to force oxidation of an unreactive alcohol.
Common alternatives to these sulfonium-based methods are oxidations with
*hypervalent iodine (e.g., Dess-Martin periodinane, 2-Iodoxybenzoic acid
2-Iodoxybenzoic acid (IBX) is an organic compound used in organic synthesis as an oxidizing agent. This periodinane is especially suited to oxidize alcohols to aldehydes. IBX is prepared from 2-iodobenzoic acid, potassium bromate, and sulfuric a ...
)
*chromium reagents (e.g., Pyridinium chlorochromate, Collins reagent)
*ruthenium oxides (e.g., Tetrapropylammonium perruthenate)
* oxoammonium species (e.g., TEMPO)
*transfer hydrogenation or hydride transfer (e.g., Oppenauer oxidation)
* MnO2, Barium manganate, 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (or DDQ) is the chemical reagent with formula C6Cl2(CN)2O2. This oxidant is useful for the dehydrogenation of alcohols, phenols, and steroid ketones. DDQ decomposes in water, but is stable in aqueous mine ...
for allylic alcohols
Categories
The sulfonium oxidations can be categorized into two groups: The methods discovered earliest rely on activated alcohols like alkyl tosylates (Kornblum oxidation
The Kornblum oxidation, named after Nathan Kornblum, is an organic oxidation reaction that converts alkyl halides and tosylates into carbonyl compounds.
Mechanism
Similar to sulfonium-based oxidation of alcohols to aldehydes reactions, the ...
) or alkyl chloroformates (from reaction of alcohols with phosgene: Barton-Kornblum) that react as electrophiles when treated with DMSO, liberating an oxygenated leaving group (e.g. ). However, the additional step for pre-activation of the alcohol and sometimes harsh reaction conditions for the nucleophilic displacement proved less convenient. Therefore, methods generating activated sulfoxides have been developed later. Depicted below is the activated sulfoxide generated during Swern oxidation 4 reacting with a secondary alcohol 5 to form alkoxysulfonium species 6.  These activated sulfoxides react as electrophiles when treated with an alcohol, expelling a leaving group that might simultaneously function as counter-ion to the alkoxysulfonium species () generated. Upon deprotonation – usually assisted by a mild base like triethylamine – the alkoxysulfonium species decomposes, yielding the aldehyde and dimethylsulfide. The latter collection contains popular oxidations like
* Swern,
* Corey-Kim,
* Parikh-Doering,
* Pfitzner-Moffatt
and also includes Albright-Goldman, Albright-Onodera (DMSO/P2O5), TFAA/DMSO (Swern) and Me2S/Cl2. Recently, SO2F2 has been proposed for generating the activated sulfoxide from DMSO.
These activated sulfoxides react as electrophiles when treated with an alcohol, expelling a leaving group that might simultaneously function as counter-ion to the alkoxysulfonium species () generated. Upon deprotonation – usually assisted by a mild base like triethylamine – the alkoxysulfonium species decomposes, yielding the aldehyde and dimethylsulfide. The latter collection contains popular oxidations like
* Swern,
* Corey-Kim,
* Parikh-Doering,
* Pfitzner-Moffatt
and also includes Albright-Goldman, Albright-Onodera (DMSO/P2O5), TFAA/DMSO (Swern) and Me2S/Cl2. Recently, SO2F2 has been proposed for generating the activated sulfoxide from DMSO.
See also
*Alcohol oxidation Alcohol oxidation is a class of organic reactions in which the alcohol functional group is converted into another functional group (e.g., aldehyde, ketone, carboxylic acid) in which carbon carries a higher oxidation state.
Through a variety of mec ...
* Oxidation with chromium(VI)-amine complexes Oxidation with chromium(VI) complexes involves the conversion of alcohols to carbonyl compounds or more highly oxidized products through the action of molecular chromium(VI) oxides and salts. The principal reagents are Collins reagent, PDC, and PCC. ...
* Oxoammonium-catalyzed oxidation
* Dess–Martin oxidation
The Dess–Martin oxidation is an organic reaction for the redox, oxidation of primary Alcohol (chemistry), alcohols to aldehydes and secondary alcohols to ketones using Dess–Martin periodinane.
It is named after the American chemists Daniel Ben ...
References
{{reflist Organic oxidation reactions