|
Parikh–Doering Oxidation
The Parikh– Doering oxidation is an oxidation reaction that transforms primary and secondary alcohols into aldehydes and ketones, respectively. The procedure uses dimethyl sulfoxide (DMSO) as the oxidant and the solvent, activated by the sulfur trioxide pyridine complex (SO3•C5H5N) in the presence of triethylamine or diisopropylethylamine as base. Dichloromethane is frequently used as a cosolvent for the reaction. Compared to other activated DMSO oxidations, the Parikh–Doering oxidation is operationally simple: the reaction can be run at non-cryogenic temperatures, often between 0 °C and room temperature, without formation of significant amounts of methyl thiomethylether side products. However, the Parikh–Doering oxidation sometimes requires a large excess of DMSO, SO3•C5H5N and/or base as well as prolonged reaction times for high conversions and yields to be obtained. The following example from the total synthesis of (–)-kumausallene by P.A. Evans and cowo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
William Von Eggers Doering
William von Eggers Doering (June 22, 1917 – January 3, 2011) was the Mallinckrodt Professor of Chemistry at Harvard University. Before Harvard, he taught at Columbia University, Columbia (1942–1952) and Yale (1952–1968). Doering was born in Fort Worth, Texas to academics Carl Rupp Doering and Antoinette Mathilde von Eggers, both of whom were professors at Texas Christian University. His maternal great-uncle was the prominent German financier and economist Hjalmar Schacht, sometime President of the ''Reichsbank'' and cabinet minister in Nazi Germany. Doering was an undergraduate at Harvard University, where he took courses with some of the leading organic chemists at the time, including Louis Fieser and Paul Bartlett. He stayed at Harvard for his graduate education, where he studied catalytic hydrogenation under Reginald Linstead, completing his PhD in 1943. Before beginning his independent career, he became famous for completing a (formal) quinine total synthesis with Ro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridinium
Pyridinium refers to the cation . It is the conjugate acid of pyridine. Many related cations are known involving substituted pyridines, e.g. picolines, lutidines, collidines. They are prepared by treating pyridine with acids. As pyridine is often used as an organic base in chemical reactions, pyridinium salts are produced in many acid-base reactions. Its Salt (chemistry), salts are often insoluble in the organic solvent, so Precipitation (chemistry), precipitation of the pyridinium leaving group complex is an indication of the progress of the reaction. Pyridinium cations are aromatic, as determined through Hückel's rule. They are isoelectronic with benzene. ''N''-Alkylpyridinium cations When the acidic proton is replaced by alkyl, the compounds are called ''N''-alkylpyridinium. A simple representative is Methylpyridinium, ''N''-methylpyridinium ([C5H5NCH3]+). These pyridinium intermediates have been used as electrophiles in synthetic organic chemistry to build dearomatized ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corey–Kim Oxidation
The Corey–Kim oxidation is an oxidation reaction used to synthesize aldehydes and ketones from primary and secondary alcohols. It is named for American chemist and Nobel Laureate Elias James Corey and Korean-American chemist Choung Un Kim. Although the Corey–Kim oxidation possesses the distinctive advantage over Swern oxidation of allowing an operation above –25 °C, it is not so commonly used due to issues with selectivity in substrates susceptible to chlorination by ''N''-chlorosuccinimide. Reaction mechanism Dimethyl sulfide (Me2S) is treated with ''N''-chlorosuccinimide (NCS), resulting in formation of an "active DMSO" species that is used for the activation of the alcohol. Addition of triethylamine to the activated alcohol leads to its oxidation to aldehyde or ketone and generation of dimethyl sulfide. In variance with other alcohol oxidation using "activated DMSO," the reactive oxidizing species is not generated by reaction of DMSO with an electro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pfitzner–Moffatt Oxidation
The Pfitzner–Moffatt oxidation, sometimes referred to as simply the Moffatt oxidation, is a chemical reaction for the oxidation of primary and secondary alcohols to aldehydes and ketones, respectively. The oxidant is a combination of dimethyl sulfoxide (DMSO) and dicyclohexylcarbodiimide (DCC). The reaction was first reported by J. Moffatt and his student K. Pfitzner in 1963. Stoichiometry and mechanism The reaction requires one equivalent each of the diimide, which is the dehydrating agent, and the sulfoxide, the oxidant: :(CH3)2SO + (CyN)2C + R2CHOH → (CH3)2S + (CyNH)2CO + R2C=O Typically the sulfoxide and diimide are used in excess. The reaction cogenerates dimethyl sulfide and a urea. Dicyclohexylurea ((CyNH)2CO) can be difficult to remove from the product. In terms of mechanism, the reaction is proposed to involve the intermediary of an sulfonium group, formed by a reaction between DMSO and the carbodiimide. : This species is highly reactive and is attacked by the alc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Swern Oxidation
The Swern oxidation, named after Daniel Swern, is a chemical reaction whereby a primary or secondary alcohol is oxidized to an aldehyde or ketone using oxalyl chloride, dimethyl sulfoxide (DMSO) and an organic base, such as triethylamine. It is one of the many oxidation reactions commonly referred to as 'activated DMSO' oxidations. The reaction is known for its mild character and wide tolerance of functional groups. The by-products are dimethyl sulfide ((CH3)2S), carbon monoxide (CO), carbon dioxide (CO2) and—when triethylamine is used as base— triethylammonium chloride (Et3NHCl). Of the volatile by-products, dimethyl sulfide has a strong, pervasive odour and carbon monoxide is acutely toxic, so the reaction and the work-up needs to be performed in a fume hood. Dimethyl sulfide is a volatile liquid (B.P. 37 °C) with an unpleasant odour at even low concentrations. Mechanism The first step of the Swern oxidation is the low-temperature reaction of DMSO, 1a, formally as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cortistatins
The cortistatins are a group of steroidal alkaloids first isolated in 2006 from the marine sponge '' Corticium simplex''. The cortistatins were first discovered in a search for naturally occurring compounds that inhibit proliferation of human umbilical vein endothelial cells ( HUVECs), with cortistatin A being the most potent compound in the class. The Shair group at Harvard along with collaborators have shown that cortistatin A is a highly potent and selective inhibitor of CDK8 and CDK19, the kinases that associate with Mediator complex. Out of 386 kinases evaluated, cortistatin A only inhibited CDK8 and CDK19, revealing that it is among the most selective kinase inhibitors. It was also shown that cortistatin A potently inhibits growth of acute myeloid leukemia cells and AML in two in vivo mouse models. Identification of dominant drug-resistant alleles of CDK8 and CDK19 demonstrate that these kinases mediate the activity of cortistatin A in AML cells. Thus, inhibition of CDK8 an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mechanism Of The Parikh-Doering Oxidation (Part 2)
Mechanism may refer to: *Mechanism (engineering), rigid bodies connected by joints in order to accomplish a desired force and/or motion transmission * Mechanism (biology), explaining how a feature is created *Mechanism (philosophy), a theory that all natural phenomena can be explained by physical causes *Mechanism (sociology), a theory that all social phenomena can be explained by the existence of a deterministic mechanism * "The Mechanism", song by Disclosure * ''The Mechanism'' (TV series), a Netflix TV series See also *Machine * Machine (mechanical) * Linkage (mechanical) * Mechanism design, the art of designing rules of a game to achieve a specific outcome * Mechanism of action, the means by which a drug exerts its biological effects * Defence mechanism, unconscious mechanisms aimed at reducing anxiety *Reaction mechanism, the sequence of reactions by which overall chemical change occurs *Antikythera mechanism The Antikythera mechanism ( ) is an Ancient Greece, Ancient ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyl Sulfide
Dimethyl sulfide (DMS) or methylthiomethane is an organosulfur compound with the formula (CH3)2S. Dimethyl sulfide is a flammable liquid that boils at and has a characteristic disagreeable odor. It is a component of the smell produced from cooking of certain vegetables, notably maize, cabbage, beetroot, and seafoods. It is also an indication of bacterial contamination in malt production and brewing. It is a breakdown product of dimethylsulfoniopropionate (DMSP), and is also produced by the bacterial metabolism of methanethiol. Occurrence and production DMS originates primarily from DMSP, a major secondary metabolite in some marine algae. DMS is the most abundant biological sulfur compound emitted to the atmosphere. Emission occurs over the oceans by phytoplankton. DMS is also produced naturally by bacterial transformation of dimethyl sulfoxide (DMSO) waste that is disposed of into sewers, where it can cause environmental odor problems. DMS is oxidized in the marine atmos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition State
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked with the double dagger ‡ symbol. As an example, the transition state shown below occurs during the SN2 reaction of bromoethane with a hydroxide anion: The activated complex of a reaction can refer to either the transition state or to other states along the reaction coordinate between reactants and products, especially those close to the transition state.Peter Atkins and Julio de Paula, ''Physical Chemistry'' (8th ed., W.H. Freeman 2006), p.809 According to the transition state theory, once the reactants have passed through the transition state configuration, they always continue to form products. History of concept The concept of a transition state has been important in many theories of the rates at which chemical reactions occ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
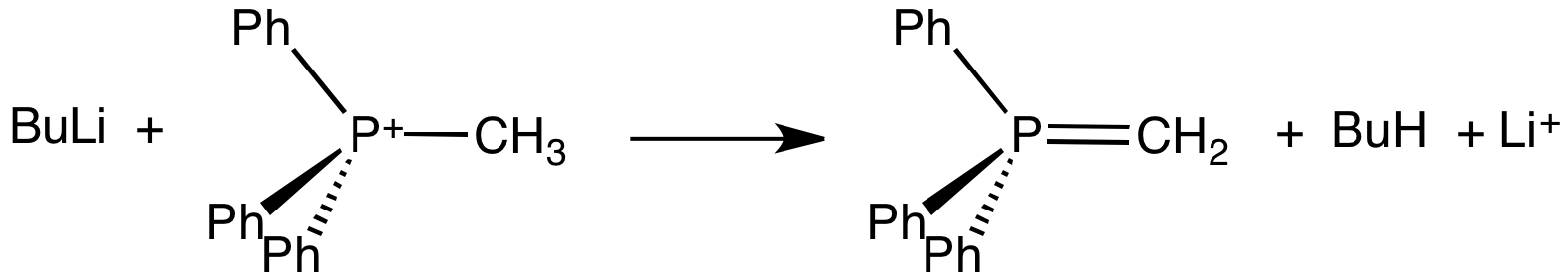
Ylide
An ylide or ylid () is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms have full octets of electrons. The result can be viewed as a structure in which two adjacent atoms are connected by both a covalent and an ionic bond; normally written X+–Y−. Ylides are thus 1,2-dipolar compounds, and a subclass of zwitterions. They appear in organic chemistry as reagents or reactive intermediates. The class name "ylide" for the compound should not be confused with the suffix "-ylide". Resonance structures Many ylides may be depicted by a multiple bond form in a resonance structure, known as the ylene form, while the actual structure lies in between both forms: : The actual bonding picture of these types of ylides is strictly zwitterionic (the structure on the right) with the strong Coulombic attraction between the " ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |