Swern oxidation on:
[Wikipedia]
[Google]
[Amazon]
The Swern oxidation, named after Daniel Swern, is a  The by-products are
The by-products are
 After addition of the alcohol 5, the chloro(dimethyl)sulfonium chloride 4 reacts with the alcohol to give the key alkoxysulfonium ion intermediate, 6. The addition of at least 2 equivalents of base — typically triethylamine — will deprotonate the alkoxysulfonium ion to give the sulfur
After addition of the alcohol 5, the chloro(dimethyl)sulfonium chloride 4 reacts with the alcohol to give the key alkoxysulfonium ion intermediate, 6. The addition of at least 2 equivalents of base — typically triethylamine — will deprotonate the alkoxysulfonium ion to give the sulfur 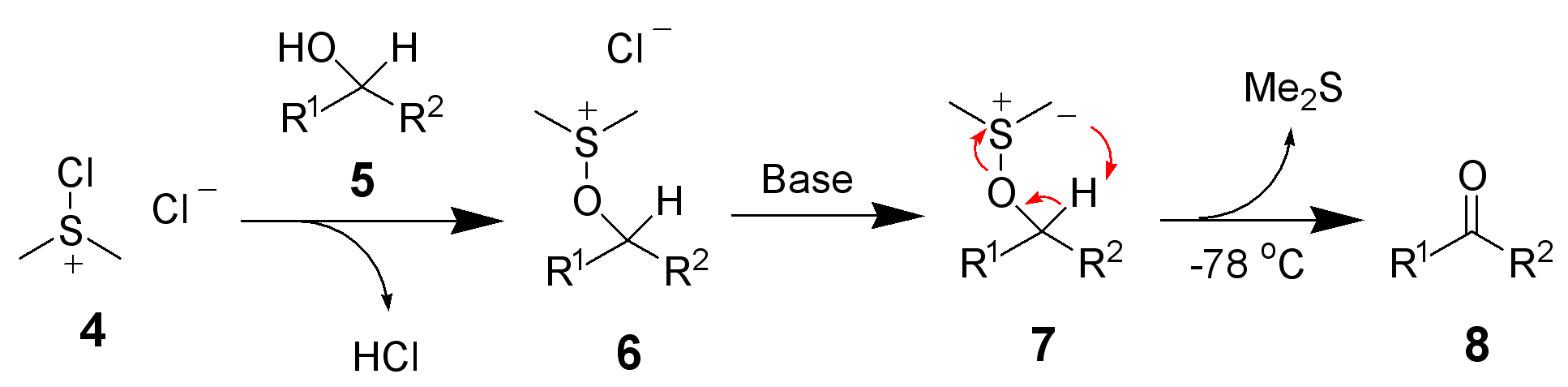

Organic Chemistry Portal
{{DEFAULTSORT:Swern Oxidation Organic oxidation reactions Name reactions
chemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and break ...
whereby a primary or secondary alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
is oxidized
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a de ...
to an aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group ...
or ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bon ...
using oxalyl chloride
Oxalyl chloride is an organic chemical compound with the formula (COCl)2. This colorless, sharp-smelling liquid, the diacyl chloride of oxalic acid, is a useful reagent in organic synthesis.
Preparation
Oxalyl chloride was first prepared in 189 ...
, dimethyl sulfoxide
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula ( CH3)2. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds ...
(DMSO) and an organic base, such as triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium, for which TEA ...
. It is one of the many oxidation reactions commonly referred to as 'activated DMSO' oxidations. The reaction is known for its mild character and wide tolerance of functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the res ...
s.
 The by-products are
The by-products are dimethyl sulfide
Dimethyl sulfide (DMS) or methylthiomethane is an organosulfur compound with the formula (CH3)2S. Dimethyl sulfide is a flammable liquid that boils at and has a characteristic disagreeable odor. It is a component of the smell produced from co ...
((CH3)2S), carbon monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
(CO), carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
(CO2) and—when triethylamine is used as base— triethylammonium chloride (Et3NHCl). Of the volatile by-products, dimethyl sulfide has a strong, pervasive odour and carbon monoxide is acutely toxic, so the reaction and the work-up needs to be performed in a fume hood. Dimethyl sulfide is a volatile liquid (B.P. 37 °C) with an unpleasant odour at even low concentrations.
Mechanism
The first step of the Swern oxidation is the low-temperature reaction of DMSO, 1a, formally asresonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillat ...
contributor 1b, with oxalyl chloride, 2. The first intermediate, 3, quickly decomposes giving off carbon dioxide and carbon monoxide and producing chloro(dimethyl)sulfonium chloride, 4.
 After addition of the alcohol 5, the chloro(dimethyl)sulfonium chloride 4 reacts with the alcohol to give the key alkoxysulfonium ion intermediate, 6. The addition of at least 2 equivalents of base — typically triethylamine — will deprotonate the alkoxysulfonium ion to give the sulfur
After addition of the alcohol 5, the chloro(dimethyl)sulfonium chloride 4 reacts with the alcohol to give the key alkoxysulfonium ion intermediate, 6. The addition of at least 2 equivalents of base — typically triethylamine — will deprotonate the alkoxysulfonium ion to give the sulfur ylide An ylide or ylid () is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both ato ...
7. In a five-membered ring transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked ...
, the sulfur ylide 7 decomposes to give dimethyl sulfide and the desired carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containin ...
compound 8.

Variations
When using oxalyl chloride as thedehydration
In physiology, dehydration is a lack of total body water, with an accompanying disruption of metabolic processes. It occurs when free water loss exceeds free water intake, usually due to exercise, disease, or high environmental temperature. Mi ...
agent, the reaction must be kept colder than −60 °C to avoid side reactions. With cyanuric chloride
Cyanuric chloride is an organic compound with the formula (NCCl)3. This white solid is the chlorinated derivative of 1,3,5-triazine. It is the trimer of cyanogen chloride. Cyanuric chloride is the main precursor to the popular but controvers ...
or trifluoroacetic anhydride instead of oxalyl chloride, the reaction can be warmed to −30 °C without side reactions. Other methods for the activation of DMSO to initiate the formation of the key intermediate 6 are the use of carbodiimides
In organic chemistry, a carbodiimide (systematic IUPAC name: methanediimine) is a functional group with the formula RN=C=NR. They are exclusively synthetic. A well known carbodiimide is dicyclohexylcarbodiimide, which is used in peptide synthesis. ...
( Pfitzner–Moffatt oxidation), a sulfur trioxide pyridine complex ( Parikh–Doering oxidation) or acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a c ...
( Albright-Goldman oxidation). The intermediate 4 can also be prepared from dimethyl sulfide and ''N''-chlorosuccinimide (the Corey–Kim oxidation).
In some cases, the use of triethylamine as the base can lead to epimerisation
In stereochemistry, an epimer is one of a pair of diastereomers. The two epimers have opposite configuration at only one stereogenic center out of at least two. All other stereogenic centers in the molecules are the same in each. Epimerization is ...
at the carbon alpha to the newly formed carbonyl. Using a bulkier base, such as diisopropylethylamine
''N'',''N''-Diisopropylethylamine, or Hünig's base, is an organic compound and an amine. It is named after the German chemist Siegfried Hünig. It is used in organic chemistry as a base. It is commonly abbreviated as DIPEA, DIEA, or ''i''-Pr2N ...
, can mitigate this side reaction.
Considerations
Dimethyl sulfide, a byproduct of the Swern oxidation, is one of the most notoriously unpleasant odors known in organic chemistry. Humans can detect this compound in concentrations as low as 0.02 to 0.1 parts per million. A simple remedy for this problem is to rinse used glassware with bleach oroxone
Potassium peroxymonosulfate is widely used as an oxidizing agent. It is the potassium salt of peroxymonosulfuric acid. Usually potassium peroxymonosulfate refers to the triple salt known as oxone.
The standard electrode potential for potassium p ...
solution, which will oxidize the dimethyl sulfide back to dimethyl sulfoxide or to dimethyl sulfone, both of which are odourless and nontoxic.
The reaction conditions allow oxidation of acid-sensitive compounds, which might decompose under the acidic oxidation conditions such as Jones oxidation
The Jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones, respectively. It is named after its discoverer, Sir Ewart Jones. The reaction was an early method for the oxidation of ...
. For example, in Thompson & Heathcock's synthesis of the sesquiterpene
Sesquiterpenes are a class of terpenes that consist of three isoprene units and often have the molecular formula C15H24. Like monoterpenes, sesquiterpenes may be cyclic or contain rings, including many unique combinations. Biochemical modifica ...
isovelleral, the final step uses the Swern protocol, avoiding rearrangement of the acid-sensitive cyclopropanemethanol moiety.

See also
*Alcohol oxidation Alcohol oxidation is a class of organic reactions in which the alcohol functional group is converted into another functional group (e.g., aldehyde, ketone, carboxylic acid) in which carbon carries a higher oxidation state.
Through a variety of mec ...
* Sulfonium-based oxidation of alcohols to aldehydes
* Pyridinium chlorochromate
Pyridinium chlorochromate (PCC) is a yellow-orange salt with the formula 5H5NHsup>+ rO3Clsup>−. It is a reagent in organic synthesis used primarily for oxidation of alcohols to form carbonyls. A variety of related compounds are known with sim ...
* Jones oxidation
The Jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones, respectively. It is named after its discoverer, Sir Ewart Jones. The reaction was an early method for the oxidation of ...
* Oppenauer oxidation
* Pfitzner–Moffatt oxidation
* Parikh–Doering oxidation
* Albright-Goldman oxidation
* Corey–Kim oxidation
* Dess–Martin periodinane
Dess–Martin periodinane (DMP) is a chemical reagent used in the Dess–Martin oxidation, oxidizing primary alcohols to aldehydes and secondary alcohols to ketones. This periodinane has several advantages over chromium- and DMSO-based oxidant ...
oxidation
* Ley oxidation
Ley may refer to:
Toponyms
* Ley (landform), name for a crag, rock or cliff in the north German language area
* Ley (crater), crater on the Moon
* Ley, Moselle, commune in France
* Ley Hill, hill in England
People
* Ley Matampi (born 1989 ...
( TPAP oxidation)
* TEMPO
In musical terminology, tempo ( Italian, 'time'; plural ''tempos'', or ''tempi'' from the Italian plural) is the speed or pace of a given piece. In classical music, tempo is typically indicated with an instruction at the start of a piece (ofte ...
oxidation
References
External links
Organic Chemistry Portal
{{DEFAULTSORT:Swern Oxidation Organic oxidation reactions Name reactions