|
Alcohol Oxidation
Alcohol oxidation is a class of organic reactions in which the alcohol functional group is converted into another functional group (e.g., aldehyde, ketone, carboxylic acid) in which carbon carries a higher oxidation state. Through a variety of mechanisms, the removal of a hydride equivalent converts a primary or secondary alcohol to an aldehyde or ketone, respectively. The oxidation of primary alcohols to carboxylic acids normally proceeds via the corresponding aldehyde, which is transformed via an aldehyde hydrate (''gem''-diol, R-CH(OH)2) by reaction with water. Thus, the oxidation of a primary alcohol at the aldehyde level without further oxidation to the carboxylic acid is possible by performing the reaction in absence of water, so that no aldehyde hydrate can be formed. Oxidation to aldehydes Oxidation of alcohols to aldehydes is partial oxidation; aldehydes are further oxidized to carboxylic acids. Conditions required for making aldehydes are heat and distillation. In aldeh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol To Aldehyde To Acid
Alcohol most commonly refers to: * Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom * Alcohol (drug), an intoxicant found in alcoholic drinks Alcohol may also refer to: Chemicals * Ethanol, one of several alcohols, commonly known as alcohol in everyday life ** Alcoholic beverage, sometimes referred to as "alcohol", any drink containing ethanol ** Surrogate alcohol, any substance containing ethanol that is intentionally consumed by humans but is not meant for human consumption * Methanol, a commodity chemical that can serve as a precursor to other chemicals * Alcohol fuel, a fuel containing alcohols * Alcohol powder, a powdered form of alcohol * Fusel alcohol, a mixture of several alcohols (chiefly amyl alcohol) produced as a by-product of alcoholic fermentation. * Alcohols (medicine), the use of alcohols in medicine ** Rubbing alcohol, a solution of denatured or isopropyl alcohol used in medicine Music * "Alcohol" (Barenaked Ladi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour. Acetone is miscible with water and serves as an important organic solvent in its own right, in industry, home, and laboratory. About 6.7 million tonnes were produced worldwide in 2010, mainly for use as a solvent and production of methyl methacrylate (and from that PMMA) as well as bisphenol A.Acetone World Petrochemicals report, January 2010Stylianos Sifniades, Alan B. Levy, "Acetone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. It is a common building block in |
Chromium Trioxide
Chromium trioxide (also known as chromium(VI) oxide or chromic anhydride) is an inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name. This compound is a dark-purple solid under anhydrous conditions, bright orange when wet and which dissolves in water concomitant with hydrolysis. Millions of kilograms are produced annually, mainly for electroplating. Chromium trioxide is a powerful oxidiser and a carcinogen. Production, structure, and basic reactions Chromium trioxide is generated by treating sodium dichromate with sulfuric acid: :H2SO4 + Na2Cr2O7 → 2 CrO3 + Na2SO4 + H2O Approximately 100,000 tonnes are produced annually by this or similar routes. The solid consists of chains of tetrahedrally coordinated chromium atoms that share vertices. Each chromium center therefore shares two oxygen centers with neighbors. Two oxygen atoms are not shared, giving an overall stoichiometry of 1:3. : The s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganese Dioxide
Manganese dioxide is the inorganic compound with the formula . This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for is for dry-cell batteries, such as the alkaline battery and the zinc–carbon battery.. is also used as a pigment and as a precursor to other manganese compounds, such as . It is used as a reagent in organic synthesis, for example, for the oxidation of allylic alcohols. is α polymorph that can incorporate a variety of atoms (as well as water molecules) in the "tunnels" or "channels" between the manganese oxide octahedra. There is considerable interest in as a possible cathode for lithium-ion batteries. Structure Several polymorphs of are claimed, as well as a hydrated form. Like many other dioxides, crystallizes in the rutile crystal structure (this polymorph is called pyrolusite or ), with three-coordinate oxide and octahedral metal centres. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
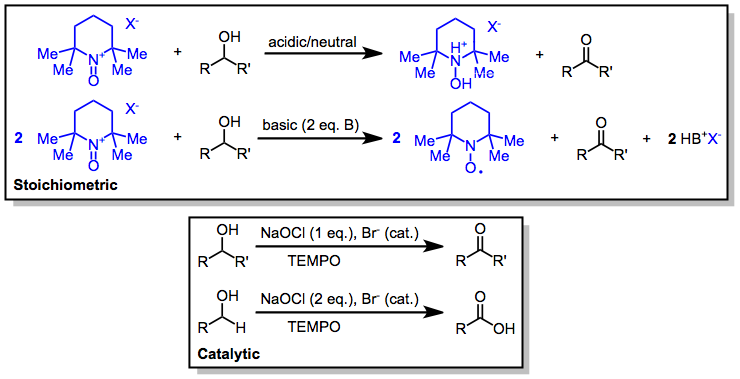
Oxoammonium-catalyzed Oxidation
Oxoammonium-catalyzed oxidation reactions involve the conversion of organic substrates to more highly oxidized materials through the action of an N-oxoammonium species. Nitroxides may also be used in catalytic amounts in the presence of a stoichiometric amount of a terminal oxidant. Bobbitt, J. M.; Bruckner, C.; Merbouh, N. '' Org. React.'' 2009, ''74'', 103. Nitroxide radical species used are either 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) or derivatives thereof. Mechanism and stereochemistry One-electron oxidation of the nitroxide produces a highly electrophilic oxoammonium species, which serves as the active oxidizing agent. The nitroxide can be used as a catalyst in conjunction with cheaper stoichiometric oxidants such as sodium hypochlorite or bis(acetoxy)iodobenzene (BAIB). Under neutral or slightly acidic conditions (in the presence of silica gel, for instance), oxidation occurs by an initial hydrogen bond between the hydroxyl group and the oxoammonium nitrogen, fol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NaOCl
Sodium hypochlorite (commonly known in a dilute solution as bleach) is an inorganic chemical compound with the formula NaOCl (or NaClO), comprising a sodium cation () and a hypochlorite anion (or ). It may also be viewed as the sodium salt of hypochlorous acid. The anhydrous compound is unstable and may decompose explosively. It can be crystallized as a pentahydrate ·5, a pale greenish-yellow solid which is not explosive and is stable if kept refrigerated. Sodium hypochlorite is most often encountered as a pale greenish-yellow dilute solution referred to as liquid bleach, which is a household chemical widely used (since the 18th century) as a disinfectant or a bleaching agent. In solution, the compound is unstable and easily decomposes, liberating chlorine, which is the active principle of such products. Sodium hypochlorite is the oldest and still most important chlorine-based bleach. Its corrosive properties, common availability, and reaction products make it a significant sa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bleach
Bleach is the generic name for any chemical product that is used industrially or domestically to remove color (whitening) from a fabric or fiber or to clean or to remove stains in a process called bleaching. It often refers specifically, to a dilute solution of sodium hypochlorite, also called "liquid bleach". Many bleaches have broad spectrum bactericidal properties, making them useful for disinfecting and sterilizing. They are used in swimming pool sanitation to control bacteria, viruses, and algae, and in many places where sterile conditions are required. They are also used in many industrial processes, notably in the bleaching of wood pulp. Bleaches also have other minor uses like removing mildew, killing weeds, and increasing the longevity of cut flowers. Bleaches work by reacting with many colored organic compounds, such as natural pigments, and turning them into colorless ones. While most bleaches are oxidizing agents (chemicals that can remove electrons from other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TEMPO
In musical terminology, tempo (Italian, 'time'; plural ''tempos'', or ''tempi'' from the Italian plural) is the speed or pace of a given piece. In classical music, tempo is typically indicated with an instruction at the start of a piece (often using conventional Italian terms) and is usually measured in beats per minute (or bpm). In modern classical compositions, a "metronome mark" in beats per minute may supplement or replace the normal tempo marking, while in modern genres like electronic dance music, tempo will typically simply be stated in BPM. Tempo may be separated from articulation and meter, or these aspects may be indicated along with tempo, all contributing to the overall texture. While the ability to hold a steady tempo is a vital skill for a musical performer, tempo is changeable. Depending on the genre of a piece of music and the performers' interpretation, a piece may be played with slight tempo rubato or drastic variances. In ensembles, the tempo is often ind ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ley Oxidation
Ley may refer to: Toponyms * Ley (landform), name for a crag, rock or cliff in the north German language area * Ley (crater), crater on the Moon * Ley, Moselle, commune in France * Ley Hill, hill in England People * Ley Matampi (born 1989), Congolese professional footballer * Ley Sander, professor of neurology and clinical epilepsy at University College London * Ley baronets, baronetcies in England and the United Kingdom ** Francis Ley (1846–1916), 1st Baronet * Bob Ley (born 1955), American sportscaster * David Ley, Canadian Geographer * Douglas Ley, American educator and politician * Duncan Ley, Australian playwright * Felix Ley (1909–1972), Roman Catholic bishop * Gary Ley (born 1956), Welsh writer * George Ley (born 1946), English footballer * Henry Ley (1887–1962), English musician * Herbert Ley, Jr., American doctor * James Ley, 1st Earl of Marlborough (1552–1629), English jurist * John Ley (1583–1662), English Puritan clergyman * John Henry Ley (17 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-Methylmorpholine N-oxide
''N''-Methylmorpholine ''N''-oxide (more correctly 4-methylmorpholine 4-oxide), NMO or NMMO is an organic compound. This heterocyclic amine oxide and morpholine derivative is used in organic chemistry as a co-oxidant and sacrificial catalyst in oxidation reactions for instance in osmium tetroxide oxidations and the Sharpless asymmetric dihydroxylation or oxidations with TPAP. NMO is commercially supplied both as a monohydrate C5H11NO2·H2O and as the anhydrous compound. The monohydrate is used as a solvent for cellulose in the lyocell process to produce cellulose fibers. Uses Solvent of cellulose NMMO monohydrate is used as a solvent in the lyocell process to produce lyocell fiber. It dissolves cellulose to form a solution called dope, and the cellulose is reprecipitated in a water bath to produce a fiber. The process is similar but not analogous to the viscose process. In the viscose process, cellulose is made soluble by conversion to its xanthate derivatives. With NMMO, cellul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrapropylammonium Perruthenate
Tetrapropylammonium perruthenate (TPAP or TPAPR) is the chemical compound described by the formula N(C3H7)4RuO4. Sometimes known as the Ley–Griffith reagent, this ruthenium compound is used as a reagent in organic synthesis. This salt consists of the tetrapropylammonium cation and the perruthenate anion, . Uses Ruthenium tetroxide is a highly aggressive oxidant, but TPAP, which is its one-electron reduced derivative, is a mild oxidizing agent for the conversion of primary alcohols to aldehydes (the Ley oxidation). Secondary alcohols are similarly oxidized to ketones. It can also be used to oxidize primary alcohols all the way to the carboxylic acid with a higher catalyst loading, larger amount of the cooxidant, and addition of two equivalents of water. In this situation, the aldehyde reacts with water to form the geminal diol hydrate, which is then oxidized again. The oxidation generates water that can be removed by adding molecular sieves. TPAP is expensive, but it can be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




.jpg)