|
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Structure
Protein structure is the three-dimensional arrangement of atoms in an amino acid-chain molecule. Proteins are polymers specifically polypeptides formed from sequences of amino acids, the monomers of the polymer. A single amino acid monomer may also be called a ''residue'' indicating a repeating unit of a polymer. Proteins form by amino acids undergoing condensation reactions, in which the amino acids lose one water molecule per reaction in order to attach to one another with a peptide bond. By convention, a chain under 30 amino acids is often identified as a peptide, rather than a protein. To be able to perform their biological function, proteins fold into one or more specific spatial conformations driven by a number of non-covalent interactions such as hydrogen bonding, ionic interactions, Van der Waals forces, and hydrophobic packing. To understand the functions of proteins at a molecular level, it is often necessary to determine their three-dimensional structure. This is t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Folding
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproducible process, a polypeptide folds into its characteristic three-dimensional structure from a random coil. Each protein exists first as an unfolded polypeptide or random coil after being translated from a sequence of mRNA to a linear chain of amino acids. At this stage the polypeptide lacks any stable (long-lasting) three-dimensional structure (the left hand side of the first figure). As the polypeptide chain is being synthesized by a ribosome, the linear chain begins to fold into its three-dimensional structure. Folding of many proteins begins even during translation of the polypeptide chain. Amino acids interact with each other to produce a well-defined three-dimensional structure, the folded protein (the right hand side of the figure), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
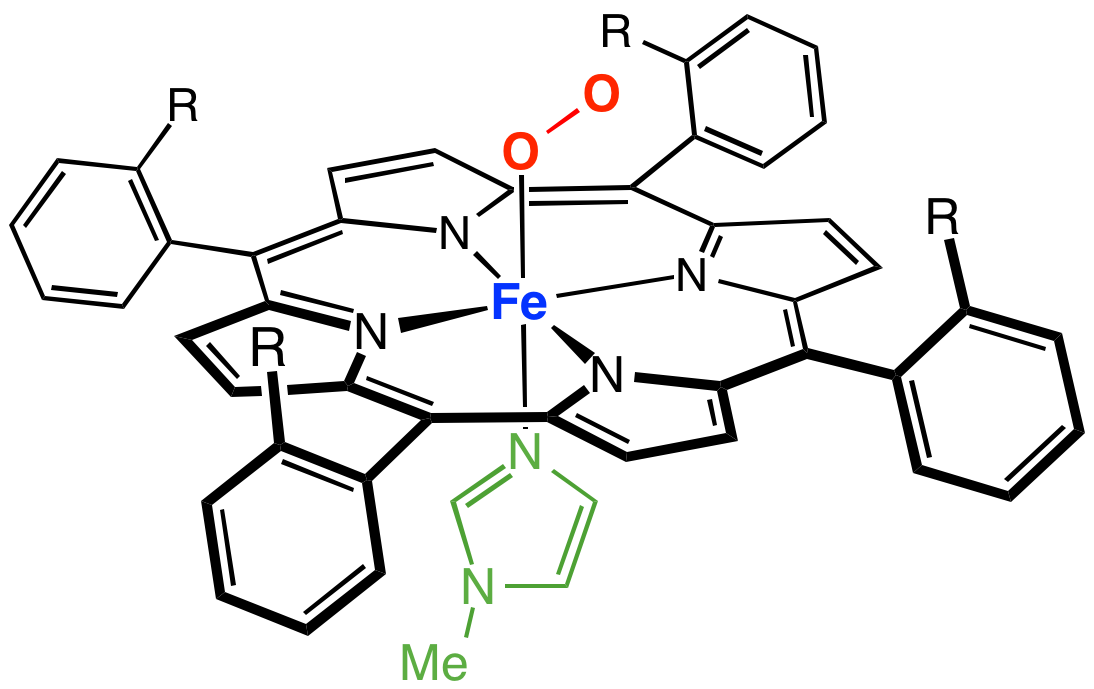
Myoglobin
Myoglobin (symbol Mb or MB) is an iron- and oxygen-binding protein found in the cardiac and skeletal muscle tissue of vertebrates in general and in almost all mammals. Myoglobin is distantly related to hemoglobin. Compared to hemoglobin, myoglobin has a higher affinity for oxygen and does not have cooperative binding with oxygen like hemoglobin does. In humans, myoglobin is only found in the bloodstream after muscle injury. (Google books link is the 2008 edition) High concentrations of myoglobin in muscle cells allow organisms to hold their breath for a longer period of time. Diving mammals such as whales and seals have muscles with particularly high abundance of myoglobin. Myoglobin is found in Type I muscle, Type II A, and Type II B; although many texts consider myoglobin not to be found in smooth muscle, this has proved erroneous: there is also myoglobin in smooth muscle cells. Myoglobin was the first protein to have its three-dimensional structure revealed by X-ray crystal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Primary Structure
Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthesis is most commonly performed by ribosomes in cells. Peptides can also be synthesized in the laboratory. Protein primary structures can be directly sequenced, or inferred from DNA sequences. Formation Biological Amino acids are polymerised via peptide bonds to form a long backbone, with the different amino acid side chains protruding along it. In biological systems, proteins are produced during translation by a cell's ribosomes. Some organisms can also make short peptides by non-ribosomal peptide synthesis, which often use amino acids other than the standard 20, and may be cyclised, modified and cross-linked. Chemical Peptides can be synthesised chemically via a range of laboratory methods. Chemical methods typically synthesise ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable atoms survive. The term is also used more generally to characterize any type of exponential (or, rarely, non-exponential) decay. For example, the medical sciences refer to the biological half-life of drugs and other chemicals in the human body. The converse of half-life (in exponential growth) is doubling time. The original term, ''half-life period'', dating to Ernest Rutherford's discovery of the principle in 1907, was shortened to ''half-life'' in the early 1950s. Rutherford applied the principle of a radioactive element's half-life in studies of age determination of rocks by measuring the decay period of radium to lead-206. Half-life is constant over the lifetime of an exponentially decaying quantity, and it is a characteristic unit for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Turnover
In cell biology, protein turnover refers to the replacement of older proteins as they are broken down within the cell. Different types of proteins have very different turnover rates. A balance between protein synthesis and protein degradation is required for good health and normal protein metabolism. More synthesis than breakdown indicates an anabolic state that builds lean tissues, more breakdown than synthesis indicates a catabolic state that burns lean tissues. According to D.S. Dunlop, protein turnover occurs in brain cells the same as any other eukaryotic cells, but that "knowledge of those aspects of control and regulation specific or peculiar to brain is an essential element for understanding brain function." Protein turnover is believed to decrease with age in all senescent organisms including humans. This results in an increase in the amount of damaged protein within the body. Protein turnover in the exercise science Four weeks of aerobic exercise has been shown to i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteolysis
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called proteases, but may also occur by intra-molecular digestion. Proteolysis in organisms serves many purposes; for example, digestive enzymes break down proteins in food to provide amino acids for the organism, while proteolytic processing of a polypeptide chain after its synthesis may be necessary for the production of an active protein. It is also important in the regulation of some physiological and cellular processes including apoptosis, as well as preventing the accumulation of unwanted or misfolded proteins in cells. Consequently, abnormality in the regulation of proteolysis can cause disease. Proteolysis can also be used as an analytical tool for studying proteins in the laboratory, and it may also be used in industry, for example in food proc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Complex
A protein complex or multiprotein complex is a group of two or more associated polypeptide chains. Protein complexes are distinct from multienzyme complexes, in which multiple catalytic domains are found in a single polypeptide chain. Protein complexes are a form of quaternary structure. Proteins in a protein complex are linked by non-covalent protein–protein interactions. These complexes are a cornerstone of many (if not most) biological processes. The cell is seen to be composed of modular supramolecular complexes, each of which performs an independent, discrete biological function. Through proximity, the speed and selectivity of binding interactions between enzymatic complex and substrates can be vastly improved, leading to higher cellular efficiency. Many of the techniques used to enter cells and isolate proteins are inherently disruptive to such large complexes, complicating the task of determining the components of a complex. Examples of protein complexes include the p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound or metallic ion that is required for an enzyme's role as a catalyst (a catalyst is a substance that increases the rate of a chemical reaction). Cofactors can be considered "helper molecules" that assist in biochemical transformations. The rates at which these happen are characterized in an area of study called enzyme kinetics. Cofactors typically differ from ligands in that they often derive their function by remaining bound. Cofactors can be divided into two types: inorganic ions and complex organic molecules called coenzymes. Coenzymes are mostly derived from vitamins and other organic essential nutrients in small amounts. (Note that some scientists limit the use of the term "cofactor" for inorganic substances; both types are included here.) Coenzymes are further divided into two types. The first is called a "prosthetic group", which consists of a coenzyme that is tightly (or even covalently) and permanently bound to a protein. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prosthetic Group
A prosthetic group is the non-amino acid component that is part of the structure of the heteroproteins or conjugated proteins, being tightly linked to the apoprotein. Not to be confused with the cofactor that binds to the enzyme apoenzyme (either a holoprotein or heteroprotein) by non-covalent binding a non-protein (non-amino acid) This is a component of a conjugated protein that is required for the protein's biological activity. The prosthetic group may be organic (such as a vitamin, sugar, RNA, phosphate or lipid) or inorganic (such as a metal ion). Prosthetic groups are bound tightly to proteins and may even be attached through a covalent bond. They often play an important role in enzyme catalysis. A protein without its prosthetic group is called an apoprotein, while a protein combined with its prosthetic group is called a holoprotein. A non-covalently bound prosthetic group cannot generally be removed from the holoprotein without denaturating the protein. Thus, the term ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Post-translational Modification
Post-translational modification (PTM) is the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus. Proteins are synthesized by ribosomes translating mRNA into polypeptide chains, which may then undergo PTM to form the mature protein product. PTMs are important components in cell signaling, as for example when prohormones are converted to hormones. Post-translational modifications can occur on the amino acid side chains or at the protein's C- or N- termini. They can extend the chemical repertoire of the 20 standard amino acids by modifying an existing functional group or introducing a new one such as phosphate. Phosphorylation is a highly effective mechanism for regulating the activity of enzymes and is the most common post-translational modification. Many eukaryotic and prokaryotic proteins also have carbohydrate molecules attached to them in a process called glycosyla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrrolysine
Pyrrolysine (symbol Pyl or O; encoded by the 'amber' stop codon UAG) is an α-amino acid that is used in the biosynthesis of proteins in some methanogenic archaea and bacteria; it is not present in humans. It contains an α-amino group (which is in the protonated – form under biological conditions), a carboxylic acid group (which is in the deprotonated –COO− form under biological conditions). Its pyrroline side-chain is similar to that of lysine in being basic and positively charged at neutral pH. Genetics Nearly all genes are translated using only 20 standard amino acid building blocks. Two unusual genetically-encoded amino acids are selenocysteine and pyrrolysine. Pyrrolysine was discovered in 2002 at the active site of methyltransferase enzyme from a methane-producing archeon, ''Methanosarcina barkeri''. This amino acid is encoded by UAG (normally a stop codon), and its synthesis and incorporation into protein is mediated via the biological machinery encoded by the ' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |