|
Phenylmethylsulfonylfluoride
In biochemistry, phenylmethylsulfonyl fluoride (PMSF) is a serine protease inhibitor (serine hydrolase inactivator) commonly used in the preparation of cell lysates. PMSF does not inactivate all serine proteases. The effective concentration of PMSF is between 0.1 - 1 mM. The half-life is short in aqueous solutions (110 min at pH 7, 55 min at pH 7.5, and 35 min at pH 8, all at 25 °C). Stock solutions are usually made up in anhydrous ethanol, isopropanol, or corn oil and diluted immediately before use. PMSF binds specifically to the active site serine residue in serine hydrolases. It does not bind to any other serine residues in the protein. This is a result of the hyperactivity of that serine residue caused by the specific environmental conditions in the enzyme's active site (catalytic triad). Because PMSF binds covalently to the enzyme, the complex can be viewed by X-ray crystallography; it can therefore be used as a chemical label to identify an essential active site serine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and metabolism. Over the last decades of the 20th century, biochemistry has become successful at explaining living processes through these three disciplines. Almost all areas of the life sciences are being uncovered and developed through biochemical methodology and research. Voet (2005), p. 3. Biochemistry focuses on understanding the chemical basis which allows biological molecules to give rise to the processes that occur within living cells and between cells,Karp (2009), p. 2. in turn relating greatly to the understanding of tissues and organs, as well as organism structure and function.Miller (2012). p. 62. Biochemistry is closely related to molecular biology, which is the study of the molecular mechanisms of biological phenomena.As ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
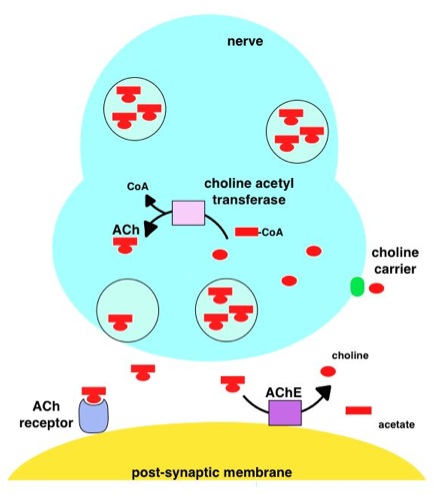
Acetylcholine Esterase
Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of acetylcholine and some other choline esters that function as neurotransmitters: : acetylcholine + H2O = choline + acetate It is found at mainly neuromuscular junctions and in chemical synapses of the cholinergic type, where its activity serves to terminate synaptic transmission. It belongs to the carboxylesterase family of enzymes. It is the primary target of inhibition by organophosphorus compounds such as nerve agents and pesticides. Enzyme structure and mechanism AChE is a hydrolase that hydrolyzes choline esters. It has a very high catalytic activity—each molecule of AChE degrades about 25,000 molecules of acetylcholine (ACh) per second, approaching the limit allowed by diffusion of the substrate. The active site of AChE comprises ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protease Inhibitors
Protease inhibitors (PIs) are medications that act by interfering with enzymes that cleave proteins. Some of the most well known are antiviral drugs widely used to treat HIV/AIDS and hepatitis C. These protease inhibitors prevent viral replication by selectively binding to viral proteases (e.g. HIV-1 protease) and blocking proteolytic cleavage of protein precursors that are necessary for the production of infectious viral particles. Protease inhibitors that have been developed and are currently used in clinical practice include: * Antiretroviral HIV-1 protease inhibitors—class stem ** Amprenavir ** Atazanavir ** Darunavir ** Fosamprenavir ** Indinavir ** Lopinavir ** Nelfinavir ** Ritonavir ** Saquinavir ** Tipranavir * Hepatitis C virus NS3/ 4A protease inhibitors—class stem ** Asunaprevir ** Boceprevir ** Grazoprevir ** Glecaprevir ** Paritaprevir ** Simeprevir ** Telaprevir * Severe acute respiratory syndrome coronavirus 2 3-chymotrypsin-like protease inhibitors ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptidase Inhibitor
In biology and biochemistry, protease inhibitors, or antiproteases, are molecules that inhibit the function of proteases (enzymes that aid the breakdown of proteins). Many naturally occurring protease inhibitors are proteins. In medicine, ''protease inhibitor'' is often used interchangeably with alpha 1-antitrypsin (A1AT, which is abbreviated PI for this reason). A1AT is indeed the protease inhibitor most often involved in disease, namely in alpha-1 antitrypsin deficiency. Classification Protease inhibitors may be classified either by the type of protease they inhibit, or by their mechanism of action. In 2004 Rawlings and colleagues introduced a classification of protease inhibitors based on similarities detectable at the level of amino acid sequence. This classification initially identified 48 families of inhibitors that could be grouped into 26 related superfamily (or clans) by their structure. According to the MEROPS database there are now 81 families of inhibitors. These f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptidase
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes (increases reaction rate or "speeds up") proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in many biological functions, including digestion of ingested proteins, protein catabolism (breakdown of old proteins), and cell signaling. In the absence of functional accelerants, proteolysis would be very slow, taking hundreds of years. Proteases can be found in all forms of life and viruses. They have independently evolved multiple times, and different classes of protease can perform the same reaction by completely different catalytic mechanisms. Hierarchy of proteases Based on catalytic residue Proteases can be classified into seven broad groups: * Serine proteases - ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MEROPS
MEROPS is an online database for peptidases (also known as proteases, proteinases and proteolytic enzymes) and their inhibitors. The classification scheme for peptidases was published by Rawlings & Barrett in 1993, and that for protein inhibitors by Rawlings ''et al.'' in 2004.Rawlings, N.D., Tolle, D.P. & Barrett, A.J. (2004) "Evolutionary families of peptidase inhibitors." ''Biochem J'' 378, 705-716. The most recent version, MEROPS 12.3, was released in September 2020. Overview The classification is based on similarities at the tertiary and primary structural levels. Comparisons are restricted to that part of the sequence directly involved in the reaction, which in the case of a peptidase must include the active site, and for a protein inhibitor the reactive site. The classification is hierarchical: sequences are assembled into families, and families are assembled into clans. Each peptidase, family, and clan has a unique identifier. Classification Family The families of pe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AEBSF
AEBSF or 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride is a water-soluble, irreversible serine protease inhibitor with a molecular weight of 239.5 Da. It inhibits proteases like chymotrypsin, kallikrein, plasmin, thrombin, and trypsin. The specificity is similar to the inhibitor PMSF, nevertheless AEBSF is more stable at low pH values. Typical usage is 0.1 - 1.0 mM. Mechanism of action Both AEBSF and PMSF are sulfonyl fluorides and are sulfonylating agents. Sulfonyl fluorides act by reacting with the hydroxy group of the active site serine residue to form a sulfonyl enzyme derivative. This derivative may be stable for long periods of time except at high pH. Use in cholesterol regulation studies AEBSF is extensively used in studies aiming to describe cholesterol regulatory genes due to its potent ability to inhibit Site-1-protease (S1P). This serine protease, located in the Golgi apparatus, is responsible for activating the sterol regulatory element-binding protei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyl Sulfoxide
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula ( CH3)2. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water. It has a relatively high boiling point. DMSO has the unusual property that many individuals perceive a garlic-like taste in the mouth after DMSO makes contact with their skin. In terms of chemical structure, the molecule has idealized Cs symmetry. It has a trigonal pyramidal molecular geometry consistent with other three-coordinate S(IV) compounds, with a nonbonded electron pair on the approximately tetrahedral sulfur atom. Synthesis and production Dimethyl sulfoxide was first synthesized in 1866 by the Russian scientist Alexander Zaytsev, who reported his findings in 1867. Dimethyl sulfoxide is produced industrially from dimethyl sulfide, a by-product of the Kraf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Median Lethal Dose
In toxicology, the median lethal dose, LD50 (abbreviation for "lethal dose, 50%"), LC50 (lethal concentration, 50%) or LCt50 is a toxic unit that measures the lethal dose of a toxin, radiation, or pathogen. The value of LD50 for a substance is the dose required to kill half the members of a tested population after a specified test duration. LD50 figures are frequently used as a general indicator of a substance's acute toxicity. A lower LD50 is indicative of increased toxicity. The test was created by J.W. Trevan in 1927. The term semilethal dose is occasionally used in the same sense, in particular with translations of foreign language text, but can also refer to a sublethal dose. LD50 is usually determined by tests on animals such as laboratory mice. In 2011, the U.S. Food and Drug Administration approved alternative methods to LD50 for testing the cosmetic drug Botox without animal tests. Conventions The LD50 is usually expressed as the mass of substance administered per unit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine Protease Inhibitor
Serpins are a superfamily of proteins with similar structures that were first identified for their protease inhibition activity and are found in all kingdoms of life. The acronym serpin was originally coined because the first serpins to be identified act on chymotrypsin-like serine proteases (serine protease inhibitors). They are notable for their unusual mechanism of action, in which they irreversibly inhibit their target protease by undergoing a large conformational change to disrupt the target's active site. This contrasts with the more common competitive mechanism for protease inhibitors that bind to and block access to the protease active site. Protease inhibition by serpins controls an array of biological processes, including coagulation and inflammation, and consequently these proteins are the target of medical research. Their unique conformational change also makes them of interest to the structural biology and protein folding research communities. The conformatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalytic Triad
A catalytic triad is a set of three coordinated amino acids that can be found in the active site of some enzymes. Catalytic triads are most commonly found in hydrolase and transferase enzymes (e.g. proteases, amidases, esterases, acylases, lipases and β-lactamases). An acid- base-nucleophile triad is a common motif for generating a nucleophilic residue for covalent catalysis. The residues form a charge-relay network to polarise and activate the nucleophile, which attacks the substrate, forming a covalent intermediate which is then hydrolysed to release the product and regenerate free enzyme. The nucleophile is most commonly a serine or cysteine amino acid, but occasionally threonine or even selenocysteine. The 3D structure of the enzyme brings together the triad residues in a precise orientation, even though they may be far apart in the sequence (primary structure). As well as divergent evolution of function (and even the triad's nucleophile), catalytic triads show some ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − form under biological conditions), and a side chain consisting of a hydroxymethyl group, classifying it as a polar amino acid. It can be synthesized in the human body under normal physiological circumstances, making it a nonessential amino acid. It is encoded by the codons UCU, UCC, UCA, UCG, AGU and AGC. Occurrence This compound is one of the naturally occurring proteinogenic amino acids. Only the L-stereoisomer appears naturally in proteins. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine. Serine was first obtained from silk protein, a particularly rich source, in 1865 by Emil Cramer. Its name is derived from the Latin for silk, ''sericum''. Serine's structure was estab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |