|
Organoiron
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to- iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. While iron adopts oxidation states from Fe(−II) through to Fe(VII), Fe(IV) is the highest established oxidation state for organoiron species. Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well. Iron(0) and more reduced states Carbonyl complexes Important iron carbonyls are the three neutral binary carbonyls, iron pentacarbonyl, diiron nonacarbonyl, and triiron dodecacarbonyl. One or more carbo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in front of oxygen (32.1% and 30.1%, respectively), forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust. In its metallic state, iron is rare in the Earth's crust, limited mainly to deposition by meteorites. Iron ores, by contrast, are among the most abundant in the Earth's crust, although extracting usable metal from them requires kilns or furnaces capable of reaching or higher, about higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BCE and the use of iron tools and weapons began to displace copper alloys, in some regions, only around 1200 BCE. That event is considered the transition from the Bronze Age to the Iron A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triiron Dodecacarbonyl
Triiron dodecarbonyl is the organoiron compound with the formula Fe3(CO)12. It is a dark green solid that sublimes under vacuum. It is soluble in nonpolar organic solvents to give intensely green solutions. Most low-nuclearity clusters are pale yellow or orange. Hot solutions of Fe3(CO)12 decompose to an iron mirror, which can be pyrophoric in air.The solid decomposes slowly in air, and thus samples are typically stored cold under an inert atmosphere. It is a more reactive source of iron(0) than iron pentacarbonyl. Synthesis It was one of the first metal carbonyl clusters synthesized. It was occasionally obtained from the thermolysis of Fe(CO)5: :3 Fe(CO)5 → Fe3(CO)12 + 3 CO Traces of the compound are easily detected because of its characteristic deep green colour. UV-photolysis of Fe(CO)5 produces Fe2(CO)9, not Fe3(CO)12. The usual synthesis of Fe3(CO)12 starts with the reaction of Fe(CO)5 with base: :3 Fe(CO)5 + (C2H5)3N + H2O → C2H5)3NHHFe3(CO)11] + 3 CO + CO2 follow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
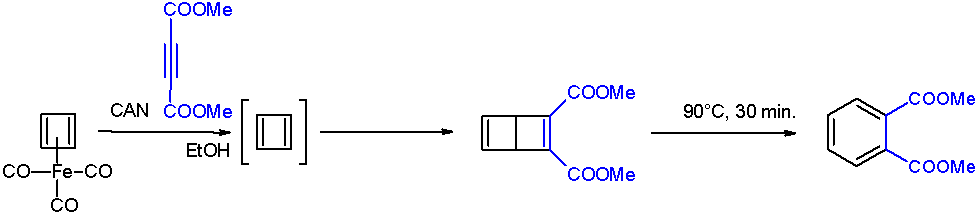
Cyclobutadieneiron Tricarbonyl
Cyclobutadieneiron tricarbonyl is an organoiron compound with the formula Fe(C4H4)(CO)3. It is a yellow solid that is soluble in organic solvents. It has been used in organic chemistry as a precursor for cyclobutadiene, which is an elusive species in the free state. Preparation and structure It was first prepared in 1965 by Pettit from 3,4-dichlorocyclobutene and diiron nonacarbonyl: :C4H4Cl2 + 2 Fe2(CO)9 → (C4H4)Fe(CO)3 + 2 Fe(CO)5 + 5 CO + FeCl2 The compound is an example of a piano stool complex. The C-C distances are 1.426 Å. Properties Oxidative decomplexation of cyclobutadiene is achieved by treating the tricarbonyl complex with ceric ammonium nitrate. The released cyclobutadiene is trapped with a quinone, which functions as a dienophile. Cyclobutadieneiron tricarbonyl displays aromaticity as evidenced by some of its reactions, which can be classified as electrophilic aromatic substitution: : It undergoes Friedel-Crafts acylation with acetyl chloride and alu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disodium Tetracarbonylferrate
Disodium tetracarbonylferrate is the organoiron compound with the formula Na2 e(CO)4 It is always used as a solvate, e.g., with tetrahydrofuran or dimethoxyethane, which bind to the sodium cation. An oxygen-sensitive colourless solid, it is a reagent in organometallic and organic chemical research. The dioxane solvated sodium salt is known as Collman's reagent, in recognition of James P. Collman, an early popularizer of its use. Structure The dianion e(CO)4sup>2− is isoelectronic with Ni(CO)4. The iron center is tetrahedral, with Na+---OCFe interactions. It is commonly used with dioxane complexed to the sodium cation. Synthesis The reagent was originally generated in situ by reducing iron pentacarbonyl with sodium amalgam. Modern synthesis use sodium naphthalene or sodium benzophenone ketyls as the reducants: :Fe(CO)5 + 2 Na → Na2 e(CO)4 + CO When a deficiency of sodium is used, the reduction affords deep yellow octacarbonyl ''di''ferrate: :2 Fe(CO)5 + 2 Na → Na2 e2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a Chemical reaction, reaction with other Chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the properties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a Catalysis, catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated and unsaturated compounds, saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces Double bond, double and Triple bond, triple bonds in hydrocarbons. Process Hydrogenation has three components, the Saturated and unsaturated compounds, unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The redox, reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protective Group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis. In many preparations of delicate organic compounds, some specific parts of their molecules cannot survive the required reagents or chemical environments. Then, these parts, or groups, must be protected. For example, lithium aluminium hydride is a highly reactive but useful reagent capable of reducing esters to alcohols. It will always react with carbonyl groups, and this cannot be discouraged by any means. When a reduction of an ester is required in the presence of a carbonyl, the attack of the hydride on the carbonyl has to be prevented. For example, the carbonyl is converted into an acetal, which does not react with hydrides. The acetal is then called a protecting group for the carbonyl. After the step involving the hydride is complete, the acet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vinyl groups. It is the simplest conjugated diene. Although butadiene breaks down quickly in the atmosphere, it is nevertheless found in ambient air in urban and suburban areas as a consequence of its constant emission from motor vehicles. The name butadiene can also refer to the isomer, 1,2-butadiene, which is a cumulated diene with structure H2C=C=CH−CH3. This allene has no industrial significance. History In 1863, the French chemist E. Caventou isolated butadiene from the pyrolysis of amyl alcohol. This hydrocarbon was identified as butadiene in 1886, after Henry Edward Armstrong isolated it from among the pyrolysis products of petroleum. In 1910, the Russian chemist Sergei Lebedev polymerized butadiene and obtained a material wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclobutadiene
Cyclobutadiene is an organic compound with the formula . It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is quite stable. Since the compound degrades by a bimolecular process, the species can be observed by matrix isolation techniques at temperatures below 35 K. It is thought to adopt a rectangular structure. Structure and reactivity The compound is the prototypical antiaromatic hydrocarbon with 4 π-electrons. It is the smallest 'n''annulene ( annulene). Its rectangular structure is the result of the Jahn–Teller effect, which distorts the molecule and lowers its symmetry, converting the triplet to a singlet ground state. The electronic states of cyclobutadiene have been explored with a variety of computational methods. The rectangular structure is consistent with the existence of two different 1,2-dideutero-1,3-cyclobutadiene valence iso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclooctadiene
A cyclooctadiene (sometimes abbreviated COD) is any of several cyclic diene with the formula (CH2)4(C2H2)2. Focusing only on cis derivatives, four isomers are possible: 1,2-, which is an allene, 1,3-, 1,4-, and 1,5-. Commonly encountered isomers are the conjugated isomer 1,3-cyclooctadiene and 1,5-cyclooctadiene, which is used as a ligand for transition metal In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...s. These dienes are colorless volatile liquids.Thomas Schiffer, Georg Oenbrink “Cyclododecatriene, Cyclooctadiene, and 4-Vinylcyclohexene” in Ullmann’s Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. References External links1,5-Cyclooctadiene Cycloalkenes Dienes Eight-membered rings {{hydrocarbon-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |