|
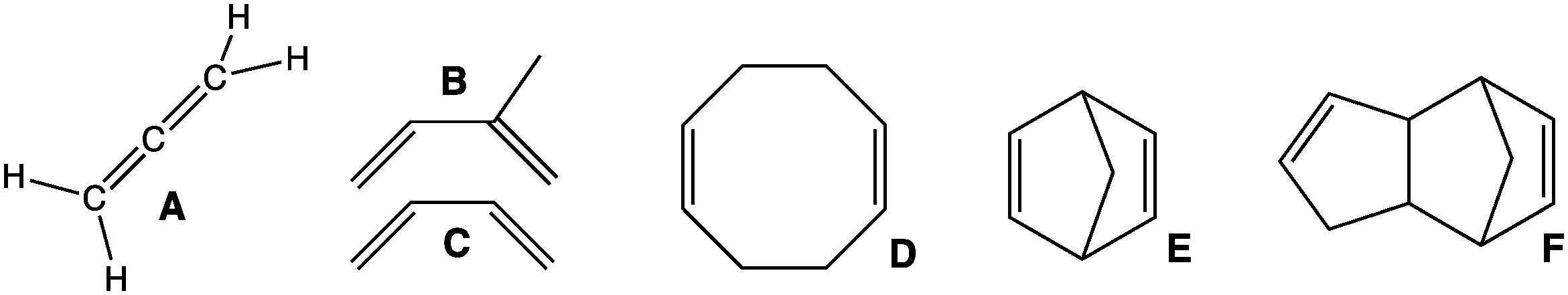
Butadiene
1,3-Butadiene () is the organic compound with the formula CH2=CH-CH=CH2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vinyl groups. It is the simplest conjugated diene. Although butadiene breaks down quickly in the atmosphere, it is nevertheless found in ambient air in urban and suburban areas as a consequence of its constant emission from motor vehicles. The name butadiene can also refer to the isomer, 1,2-butadiene, which is a cumulated diene with structure H2C=C=CH−CH3. This allene has no industrial significance. History In 1863, French chemist E. Caventou isolated butadiene from the pyrolysis of amyl alcohol. This hydrocarbon was identified as butadiene in 1886, after Henry Edward Armstrong isolated it from among the pyrolysis products of petroleum. In 1910, the Russian chemist Sergei Lebedev polymerized butadiene and obtained a material ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Walter Bock
Walter Bock (20 January 1895 – 25 October 1948)Death record Nr. 3271/Köln I for Ludwig Walter Robert Bock of Oct. 26, 1948, Landesarchiv NRW, Duisburg was a German chemist who developed styrene-butadiene , styrene-butadiene copolymer by emulsion polymerization as a synthetic rubber (SBR). Early life Walter Bock was born on January 10, 1895, in the small village of Wenzen (now part of Einbeck) in the Duchy of Brunswick. He was the fourth of nine children. His father, Wilhelm Bock, was the sole teacher in Wenzen. From 1905 to 1914 Bock attended high school in Brunswick. Immediately after graduation he joined the army and served as an officer in World War I. He commanded an infantry company until he was wounded in July 1918. In October 1918 he began studying chemistry. After receiving his Ph.D. from the University of Göttingen in October 1921, Bock found employment as chemist at the Köln Rottweil AG in Premnitz. In the fall of 1924 Bock joined the Dr. Zellner laboratories in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sergei Vasilyevich Lebedev
Sergei Vasilievich Lebedev (; 13 July 1874 – 2 May 1934) was a Russian/Soviet chemist and the inventor of polybutadiene synthetic rubber, the first commercially viable and mass-produced type of synthetic rubber. Biography Lebedev was born in 1874 in Lublin and went to school in Warsaw. In 1900, he graduated from St. Petersburg University and found work at the Petersburg Margarine Factory. Starting in 1902, Lebedev moved from university to university in Russia, starting at the Saint-Petersburg Institute for Railroad Engineering. In 1904, he returned to St. Petersburg University to work under Alexey Favorsky ( Stalin Prize, 1941, for contributions to the manufacture of synthetic rubber). In 1905, he married his second wife, the artist Anna Ostroumova-Lebedeva. In 1915, Lebedev was appointed Professor at the Women's Pedagogical Institute in St. Petersburg. After 1916, he was a Professor of the Saint Petersburg Academy for Military Medicine. In 1925, he became the leade ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroprene
Chloroprene (IUPAC name 2-chlorobuta-1,3-diene) is a chemical compound with the molecular formula CH2=CCl−CH=CH2. Chloroprene is a colorless volatile liquid, almost exclusively used as a monomer for the production of the polymer polychloroprene, better known as neoprene, a type of synthetic rubber. History Although it may have been discovered earlier, chloroprene was largely developed by DuPont during the early 1930s, specifically with the formation of neoprene in mind. The chemists Elmer K. Bolton, Wallace Carothers, Arnold Collins and Ira Williams are generally accredited with its development and commercialisation although the work was based upon that of Julius Arthur Nieuwland, with whom they collaborated. Production Chloroprene is produced in three steps from 1,3-butadiene: (i) chlorination, (ii) isomerization of part of the product stream, and (iii) dehydrochlorination of 3,4-dichlorobut-1-ene. Chlorine adds to 1,3-butadiene to afford a mixture of 3,4-dichlorobut-1-e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conjugated Diene
In organic chemistry, a diene ( ); also diolefin, ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nomenclature. As a subunit of more complex molecules, dienes occur in naturally occurring and synthetic chemicals and are used in organic synthesis. Conjugated dienes are widely used as monomers in the polymer industry. Polyunsaturated fats are of interest to nutrition. Classes Dienes can be divided into three classes, depending on the relative location of the double bonds: #Cumulated dienes have the double bonds sharing a common atom. The result is more specifically called an allene. #Conjugated dienes have conjugated double bonds separated by one single bond. Conjugated dienes are more stable than other dienes because of resonance. #Unconjugated dienes have the double bonds separated by two or more single bonds. They are usually less stabl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diene
In organic chemistry, a diene ( ); also diolefin, ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nomenclature. As a subunit of more complex molecules, dienes occur in naturally occurring and synthetic chemicals and are used in organic synthesis. Conjugated dienes are widely used as monomers in the polymer industry. Polyunsaturated fats are of interest to nutrition. Classes Dienes can be divided into three classes, depending on the relative location of the double bonds: #Cumulated dienes have the double bonds sharing a common atom. The result is more specifically called an allene. #Conjugated dienes have conjugated double bonds separated by one single bond. Conjugated dienes are more stable than other dienes because of resonance. #Unconjugated dienes have the double bonds separated by two or more single bonds. They are usually less ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Rubber
A synthetic rubber is an artificial elastomer. They are polymers synthesized from petroleum byproducts. About of rubber is produced annually in the United States, and of that amount two thirds are synthetic. Synthetic rubber, just like natural rubber, has many uses in the automotive industry for tires, door and window profiles, seals such as O-rings and gaskets, hoses, belts, matting, and flooring. They offer a different range of physical and chemical properties which can improve the reliability of a given product or application. Synthetic rubbers are superior to natural rubbers in two major respects: thermal stability, and resistance to oils and related compounds. They are more resistant to oxidizing agents, such as oxygen and ozone which can reduce the life of products like tires. History The expanded use of bicycles, and particularly their pneumatic tires, starting in the 1890s, created increased demand for rubber. In 1909, a team headed by Fritz Hofmann, working at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkenes
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins. The International Union of Pure and Applied Chemistry (IUPAC) Preferred IUPAC name, recommends using the name "alkene" only for Open-chain compound, acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for Cyclic compound, cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with ''n'' being a >1 natural number (which is two hydrogens less than the corresponding alkane). When ''n'' is four or more, isomers are possible, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoprene
Isoprene, or 2-methyl-1,3-butadiene, is a common volatile organic compound with the formula CH2=C(CH3)−CH=CH2. In its pure form it is a colorless volatile liquid. It is produced by many plants and animals (including humans) and its polymers are the main component of natural rubber. History and etymology Charles Greville Williams, C. G. Williams named the compound in 1860 after obtaining it from the pyrolysis of natural rubber. He correctly deduced the mass shares of carbon and hydrogen (but arrived at an incorrect formula C10H8 because the modern atomic weight of carbon was not adopted until the Karlsruhe Congress held later that year). He did not specify the reasons for the name, but it is hypothesized that it came from "propylene" with which isoprene shares some physical and chemical properties. The first one to observe recombination of isoprene into rubber-like substance was in 1879, and William A. Tilden identified its structure five years later. Natural occurrences Is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carcinogen
A carcinogen () is any agent that promotes the development of cancer. Carcinogens can include synthetic chemicals, naturally occurring substances, physical agents such as ionizing and non-ionizing radiation, and biologic agents such as viruses and bacteria. Most carcinogens act by creating mutations in DNA that disrupt a cell's normal processes for regulating growth, leading to uncontrolled cellular proliferation. This occurs when the cell's DNA repair processes fail to identify DNA damage allowing the defect to be passed down to daughter cells. The damage accumulates over time. This is typically a multi-step process during which the regulatory mechanisms within the cell are gradually dismantled allowing for unchecked cellular division. The specific mechanisms for carcinogenic activity is unique to each agent and cell type. Carcinogens can be broadly categorized, however, as activation-dependent and activation-independent which relate to the agent's ability to engage dir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butane
Butane () is an alkane with the formula C4H10. Butane exists as two isomers, ''n''-butane with connectivity and iso-butane with the formula . Both isomers are highly flammable, colorless, easily liquefied gases that quickly vaporize at room temperature and pressure. Butanes are a trace components of natural gases (NG gases). The other hydrocarbons in NG include propane, ethane, and especially methane, which are more abundant. Liquefied petroleum gas is a mixture of propane and some butanes. The name butane comes from the root but- (from butyric acid, named after the Greek word for butter) and the suffix -ane (for organic compounds). History The first synthesis of butane was accidentally achieved by British chemist Edward Frankland in 1849 from ethyl iodide and zinc, but he had not realized that the ethyl radical dimerized and misidentified the substance. It was discovered in crude petroleum in 1864 by Edmund Ronalds, who was the first to describe its proper ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of UN Numbers 1001 To 1100
UN numbers from UN1001 to UN1100 as assigned by the United Nations Committee of Experts on the Transport of Dangerous Goods are as follows: __NOTOC__ UN 1001 to UN 1100 n.o.s. = ''not otherwise specified'' meaning a collective entry to which substances, mixtures, solutions or articles may be assigned if a) they are not mentioned by name in ''3.2 Dangerous Goods List'' AND b) they exhibit chemical, physical and/or dangerous properties corresponding to the Class, classification code, packing group and the name and description of the n.o.s. entry See also * Lists of UN numbers References External linksADR Dangerous Goods cited on 2 June 2015.UN Dangerous Goods List from 2015 cited on 2 June 2015.UN Dangerous Goods List from 2013 cited on 2 June 2015. {{UN number list navbox Lists of UN numbers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
British Empire
The British Empire comprised the dominions, Crown colony, colonies, protectorates, League of Nations mandate, mandates, and other Dependent territory, territories ruled or administered by the United Kingdom and its predecessor states. It began with the English overseas possessions, overseas possessions and trading posts established by Kingdom of England, England in the late 16th and early 17th centuries, and colonisation attempts by Kingdom of Scotland, Scotland during the 17th century. At its height in the 19th and early 20th centuries, it became the List of largest empires, largest empire in history and, for a century, was the foremost global power. By 1913, the British Empire held sway over 412 million people, of the world population at the time, and by 1920, it covered , of the Earth's total land area. As a result, Westminster system, its constitutional, Common law, legal, English language, linguistic, and Culture of the United Kingdom, cultural legacy is widespread. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |