Organoiron on:
[Wikipedia]
[Google]
[Amazon]
Organoiron chemistry is the


 Compounds of the type η3-allyl)Fe(CO)4sup>+X− are
Compounds of the type η3-allyl)Fe(CO)4sup>+X− are

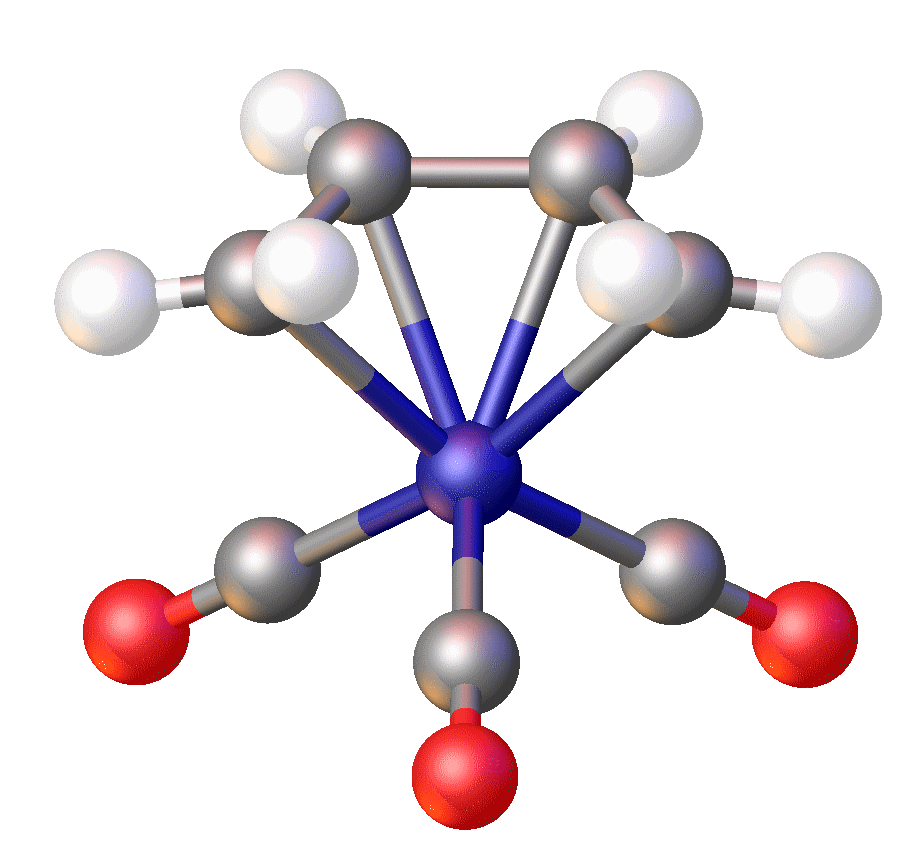
 In Fe(norbornyl)4, Fe(IV) is stabilized by an alkyl ligand that resists
In Fe(norbornyl)4, Fe(IV) is stabilized by an alkyl ligand that resists
chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
of iron compounds containing a carbon-to- iron chemical bond. Organoiron compounds are relevant in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
as reagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
s such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. While iron adopts oxidation states from Fe(−II) through to Fe(VII), Fe(IV) is the highest established oxidation state for organoiron species. Although iron is generally less active in many catalytic applications, it is less expensive and "greener Greener is a surname. Notable people with the surname include:
* Bob Greener (1899–1970), English professional footballer
* Christopher Greener (born 1943), United Kingdom's tallest human
* Matthew Greener, British musician
* Richard Theodore Gre ...
" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl Cyclopentadienyl can refer to
*Cyclopentadienyl anion, or cyclopentadienide,
**Cyclopentadienyl ligand
*Cyclopentadienyl radical, •
*Cyclopentadienyl cation,
See also
*Pentadienyl
In organic chemistry, pentadienyl refers to the organic radic ...
, but hard ligands such as amines are employed as well.
Iron(0) and more reduced states

Carbonyl complexes
Important iron carbonyls are the three neutral binary carbonyls, iron pentacarbonyl, diiron nonacarbonyl, and triiron dodecacarbonyl. One or more carbonyl ligands in these compounds can be replaced by a variety of other ligands including alkenes and phosphines. An iron(-II) complex, disodium tetracarbonylferrate (Na2 e(CO)4, also known as "Collman's Reagent," is prepared by reducing iron pentacarbonyl with metallic sodium. The highly nucleophilic anionic reagent can be alkylated and carbonylated to give the acyl derivatives that undergo protonolysis to afford aldehydes: :LiFe(CO)4(C(O)R) + H+ → RCHO (+ iron containing products) Similar iron acyls can be accessed by treating iron pentacarbonyl with organolithium compounds: :ArLi + Fe(CO)5 → LiFe(CO)4C(O)R In this case, the carbanion attacks a CO ligand. In a complementary reaction, Collman's reagent can be used to convert acyl chlorides to aldehydes. Similar reactions can be achieved with Fe(CO)4sup>− salts.Alkene-Fe(0)-CO derivatives

Monoalkenes
Iron pentacarbonyl reacts photochemically with alkenes to give Fe(CO)4(alkene).Diene-Fe(0)-CO derivatives
Iron diene complexes are usually prepared from Fe(CO)5 or Fe2(CO)9. Derivatives are known for common dienes like cyclohexadiene, norbornadiene andcyclooctadiene
A cyclooctadiene (sometimes abbreviated COD) is any of several cyclic diene with the formula (CH2)4(C2H2)2. Focusing only on cis derivatives, four isomers are possible: 1,2-, which is an allene, 1,3-, 1,4-, and 1,5-. Commonly encountered isomers ar ...
, but even cyclobutadiene can be stabilized. In the complex with butadiene, the diene adopts a cis-conformation. Iron carbonyls are potential protective groups for dienes, shielding them from hydrogenations and Diels-Alder reactions. Cyclobutadieneiron tricarbonyl is prepared from 3,4-dichlorocyclobutene and Fe2(CO)9.
Cyclohexadienes, many derived from Birch reduction of aromatic compounds, form derivatives (diene)Fe(CO)3. The affinity of the Fe(CO)3 unit for conjugated dienes is manifested in the ability of iron carbonyls catalyse the isomerisation
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomeriz ...
s of 1,5-cyclooctadiene
Cycloocta-1,5-diene is a cyclic hydrocarbon with the chemical formula , specifically .
There are three configurational isomers with this structure, that differ by the arrangement of the four C–C single bonds adjacent to the double bonds. Each ...
to 1,3-cyclooctadiene. Cyclohexadiene complexes undergo hydride abstraction to give cyclohexadienyl cations, which add nucleophiles. Hydride abstraction from cyclohexadiene iron(0) complexes gives ferrous derivatives.
The enone complex (benzylideneacetone)iron tricarbonyl
(Benzylideneacetone)iron tricarbonyl is the organoiron compound with the formula (CHCH=CHC(O)CH)Fe(CO). It is a reagent for transferring the Fe(CO) unit. This red-colored compound is commonly abbreviated (bda)Fe(CO).
Structure and bonding
(bda)Fe ...
serves as a source of the Fe(CO)3 subunit and is employed to prepare other derivatives. It is used similarly to Fe2(CO)9.
Alkyne-Fe(0)-CO derivatives
Alkynes react with iron carbonyls to give a large variety of derivatives. Derivatives includeferrole
In organoiron chemistry, a ferrole is a type of diiron complex containing the (OC)3FeC4R4 heterocycle that is pi-bonded to a Fe(CO)3 group. These compounds have Fe-Fe bonds (ca. 252 pm) and semi-bridging CO ligands (Fe-C distances = 178, 251 ...
s (Fe2(C4R4)(CO)6), (p-quinone
The quinones are a class of organic compounds that are formally "derived from aromatic compounds
)Fe(CO)3, (cyclobutadiene)Fe(CO)3 and many others.
uch as benzene or naphthalene
Uch ( pa, ;
ur, ), frequently referred to as Uch Sharīf ( pa, ;
ur, ; ''"Noble Uch"''), is a historic city in the southern part of Pakistan's Punjab province. Uch may have been founded as Alexandria on the Indus, a town founded by Alexand ...
by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double ...Tri- and polyene Fe(0) complexes
Stable iron-containing complexes with and without CO ligands are known for a wide variety of polyunsaturated hydrocarbons, e.g. cycloheptatriene, azulene, and bullvalene. In the case of cyclooctatetraene (COT), derivatives include Fe(COT)2, Fe3(COT)3, and several mixed COT-carbonyls (e.g. Fe(COT)(CO)3 and Fe2(COT)(CO)6). 144px, Bis(cyclooctatetraene)iron is an Fe(0) complex lacking CO ligands.Iron(I) and iron(II)
As Fe(II) is a common oxidation state for Fe, many organoiron(II) compounds are known. Fe(I) compounds often feature Fe-Fe bonds, but exceptions occur, such as e(anthracene)2sup>−. :
Ferrocene and its derivatives
The rapid growth of organometallic chemistry in the 20th century can be traced to the discovery offerrocene
Ferrocene is an organometallic compound with the formula . The molecule is a complex consisting of two cyclopentadienyl rings bound to a central iron atom. It is an orange solid with a camphor-like odor, that sublimes above room temperature, a ...
, a very stable compound which foreshadowed the synthesis of many related sandwich compounds. Ferrocene is formed by reaction of sodium cyclopentadienide
Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion. Sodium cyclopentadienide is a colorless solid, although samples often are pi ...
with iron(II) chloride:
:2 NaC5H5 + FeCl2 → Fe(C5H5)2 + 2 NaCl
Ferrocene displays diverse reactivity localized on the cyclopentadienyl ligands, including Friedel–Crafts reactions and lithation. Some electrophilic functionalization reactions, however, proceed via initial attack at the Fe center to give the bent p2Fe–Zsup>+ species (which are formally Fe(IV)). For instance, HF:PF5 and Hg(OTFA)2, give isolable or spectroscopically observable complexes p2Fe–Hsup>+PF6– and Cp2Fe+–Hg–(OTFA)2, respectively.
Ferrocene is also a structurally unusual scaffold as illustrated by the popularity of ligands such as 1,1'-bis(diphenylphosphino)ferrocene
1,1-Bis(diphenylphosphino)ferrocene, commonly abbreviated dppf, is an organophosphorus compound commonly used as a ligand in homogeneous catalysis. It contains a ferrocene moiety in its backbone, and is related to other bridged diphosphines such ...
, which are useful in catalysis. Treatment of ferrocene with aluminium trichloride and benzene gives the cation pFe(C6H6)sup>+. Oxidation of ferrocene gives the blue 17e species ferrocenium. Derivatives of fullerene can also act as a highly substituted cyclopentadienyl ligand.
Fp2, Fp−, and Fp+ and derivatives
Fe(CO)5 reacts withcyclopentadiene
Cyclopentadiene is an organic compound with the chemical formula, formula C5H6.LeRoy H. Scharpen and Victor W. Laurie (1965): "Structure of cyclopentadiene". ''The Journal of Chemical Physics'', volume 43, issue 8, pages 2765-2766. It is often ab ...
to give the dinuclear Fe(I) species cyclopentadienyliron dicarbonyl dimer
Cyclopentadienyliron dicarbonyl dimer is an organometallic compound with the formula ''η''5-C5H5)Fe(CO)2sub>2, often abbreviated to Cp2Fe2(CO)4, pFe(CO)2sub>2 or even Fp2, with the colloquial name "fip dimer". It is a dark reddish-purple crysta ...
( eCp(CO)2sub>2), often abbreviated as Fp2. Pyrolysis of Fp2 gives the cuboidal cluster eCp(CO)sub>4.
Very hindered substituted cyclopentadienyl ligands can give an isolable monomeric Fe(I) species. For example, Cpi-PrFe(CO)2 (Cpi-Pr = i-Pr5C5) has been characterized crystallographically.
Reduction of Fp2 with sodium gives "NaFp", containing a potent nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
and precursor to many derivatives of the type CpFe(CO)2R. The derivative pCH2S(CH3)2sup>+ has been used in cyclopropanations. The complex Cp(CO2)Fe+(η2-vinyl ether Vinyl ether may refer to:
* Any enol ether
* Divinyl ether
Divinyl ether is the organic compound with the formula O(CH=CH2)2. It is a colorless, volatile liquid that has mainly been of interest as an inhalation anesthetic. It is prepared by ...
]+ is a masked vinyl cation.
Fp-R compounds are prochiral, and studies have exploited the chiral derivatives CpFe(PPh3)(CO)acyl.
Alkyl, allyl, and aryl compounds
The simple peralkyl and peraryl complexes of iron are less numerous than are the Cp and CO derivatives. One example istetramesityldiiron
Tetramesityldiiron is an organoiron compound with the formula Fe2(C6H2(CH3)3)4. It is a red, air-sensitive solid that is used as a precursor to other iron complexes. It adopts a Molecular symmetry, centrosymmetric structure. The complex is a Lewis ...
.
 Compounds of the type η3-allyl)Fe(CO)4sup>+X− are
Compounds of the type η3-allyl)Fe(CO)4sup>+X− are allyl cation
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . ...
synthons in allylic substitution. In contrast, compounds of the type η5-C5H5)Fe(CO)2(CH2CH=CHR)possessing η1-allyl groups are analogous to main group allylmetal species (M = B, Si, Sn, etc.) and react with carbon electrophiles to give allylation products with SE2′ selectivity. Similarly, allenyl(cyclopentadienyliron) dicarbonyl complexes exhibit reactivity analogous to main group allenylmetal species and serve as nucleophilic propargyl synthons.
Sulfur and phosphorus derivatives
Complexes of the type Fe2(SR)2(CO)6 and Fe2(PR2)2(CO)6 form, usually by the reaction of thiols and secondary phosphines with iron carbonyls. The thiolates can also be obtained from the tetrahedrane Fe2S2(CO)6.Iron(III)
Some organoiron(III) compounds are prepared by oxidation of organoiron(II) compounds. A long-known example being ferrocenium C5H5)2Fesup>+. Organoiron(III) porphyrin complexes are numerous.
Iron(IV)
beta-hydride elimination
β-Hydride elimination is a reaction in which an alkyl group bonded to a metal centre is converted into the corresponding metal-bonded hydride and an alkene. The alkyl must have hydrogens on the β-carbon. For instance butyl groups can undergo th ...
. Surprisingly, FeCy4, which is susceptible to beta-hydride elimination, has also been isolated and crystallographically characterized and is stable at –20 °C. The unexpected stability was attributed to stabilizing dispersive forces as well as conformational effects that disfavor beta-hydride elimination.
Two-electron oxidation of decamethylferrocene
Decamethylferrocene or bis(pentamethylcyclopentadienyl)iron(II) is a chemical compound with formula or . It is a sandwich compound, whose molecule has an iron(II) cation attached by coordination bonds between two pentamethylcyclopentadienyl ani ...
gives the dication e(C5Me5)2sup>2+, which forms a carbonyl complex, e(C5Me5)2(CO)SbF6)2.
Organoiron compounds in organic synthesis and homogeneous catalysis
In industrial catalysis, iron complexes are seldom used in contrast to cobalt and nickel. Because of low cost and low toxicity of its salts, iron is attractive as a stoichiometric reagent. Some areas of investigation include: * Hydrogenation and reduction, example catalyst Knölker complex. *Cross-coupling reaction
In organic chemistry, a cross-coupling reaction is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = ...
s. Iron compounds such as Fe( acac)3 catalyze a wide range of cross-coupling reaction
In organic chemistry, a cross-coupling reaction is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = ...
s with one substrate an aryl or alkyl Grignard and the other substrate an aryl, alkenyl
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
(vinyl), or acyl organohalide. In the related Kumada coupling the catalysts are based on palladium and nickel.
*Complexes derived from Schiff bases are active catalysts for olefin polymerization.Allan, L. E. N.; Shaver, M. P.; White, A. J. P. and Gibson, V. C., "Correlation of Metal Spin-State in alpha-Diimine Iron Catalysts with Polymerization Mechanism", Inorg. Chem., 2007, 46, 8963-8970.
Biochemistry
In the area ofbioorganometallic chemistry
Bioorganometallic chemistry is the study of biologically active molecules that contain carbon directly bonded to metals or metalloids. The importance of main-group and transition-metal centers has long been recognized as important to the function o ...
, organoiron species are found at the active sites of the three hydrogenase enzymes as well as carbon monoxide dehydrogenase.
Further reading
*References
{{ChemicalBondsToCarbon