|
Allyl Cation
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "". The term allyl applies to many compounds related to , some of which are of practical or of everyday importance, for example, allyl chloride. Allylation is any chemical reaction that adds an allyl group to a substrate. Nomenclature A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site. A group attached at this site is sometimes described as allylic. Thus, "has an allylic hydroxyl group". Allylic C−H bonds are about 15% weaker than the C−H bonds in ordinary sp3 carbon centers and are thus more reactive. Benzylic and allylic are related in terms of structure, bond strength, and reactivity. Other reac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allyl
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "". The term allyl applies to many compounds related to , some of which are of practical or of everyday importance, for example, allyl chloride. Allylation is any chemical reaction that adds an allyl group to a substrate. Nomenclature A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site. A group attached at this site is sometimes described as allylic. Thus, "has an allylic hydroxyl group". Allylic C−H bonds are about 15% weaker than the C−H bonds in ordinary sp3 carbon centers and are thus more reactive. Benzylic and allylic are related in terms of structure, bond strength, and reactivity. Other re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allylic Oxidation
Organoselenium compounds (or seleno-organic) are chemical compounds containing carbon-to-selenium chemical bonds. Organoselenium chemistry is the corresponding science exploring their properties and reactivity. Selenium belongs with oxygen and sulfur to the group 16 elements or chalcogens, and similarities in chemistry are to be expected. Organoselenium compounds are found at trace levels in ambient waters, soils and sediments. Selenium can exist with oxidation state −2, +2, +4, +6. Se(II) is the dominant form in organoselenium chemistry. Down the group 16 column, the bond strength becomes increasingly weaker (234 kJ/ mol for the C−Se bond and 272 kJ/mol for the C−S bond) and the bond lengths longer (C−Se 198 pm, C−S 181 pm and C−O 141 pm). Selenium compounds are more nucleophilic than the corresponding sulfur compounds and also more acidic. The p''K''a values of XH2 are 16 for oxygen, 7 for sulfur and 3.8 for selenium. In contrast to sulfoxides, the corresponding sel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbanion
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form). Formally, a carbanion is the conjugate base of a carbon acid: :R3CH\, + \ddot^- -> \mathbf + HB where B stands for the base. The carbanions formed from deprotonation of alkanes (at an sp3 carbon), alkenes (at an sp2 carbon), arenes (at an sp2 carbon), and alkynes (at an sp carbon) are known as alkyl, alkenyl (vinyl), aryl, and alkynyl (acetylide) anions, respectively. Carbanions have a concentration of electron density at the negatively charged carbon, which, in most cases, reacts efficiently with a variety of electrophiles of varying strengths, including carbonyl groups, imines/ iminium salts, halogenating reagents (e.g., ''N''-bromosuccinimide and diiodine), and proton donors. A carbanion is one of several reactive intermediates in organic chemistry. In organic synthesis, organolithium reagents a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Free Radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing. Ageing Ailments of unknown cause Biogerontology Biological processes Causes of death Cellular processes Gerontology Life extension Metabolic disorders Metabolism Old age Time in life Wikipedia categories named after diseases and disorders {{CatAutoTOC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
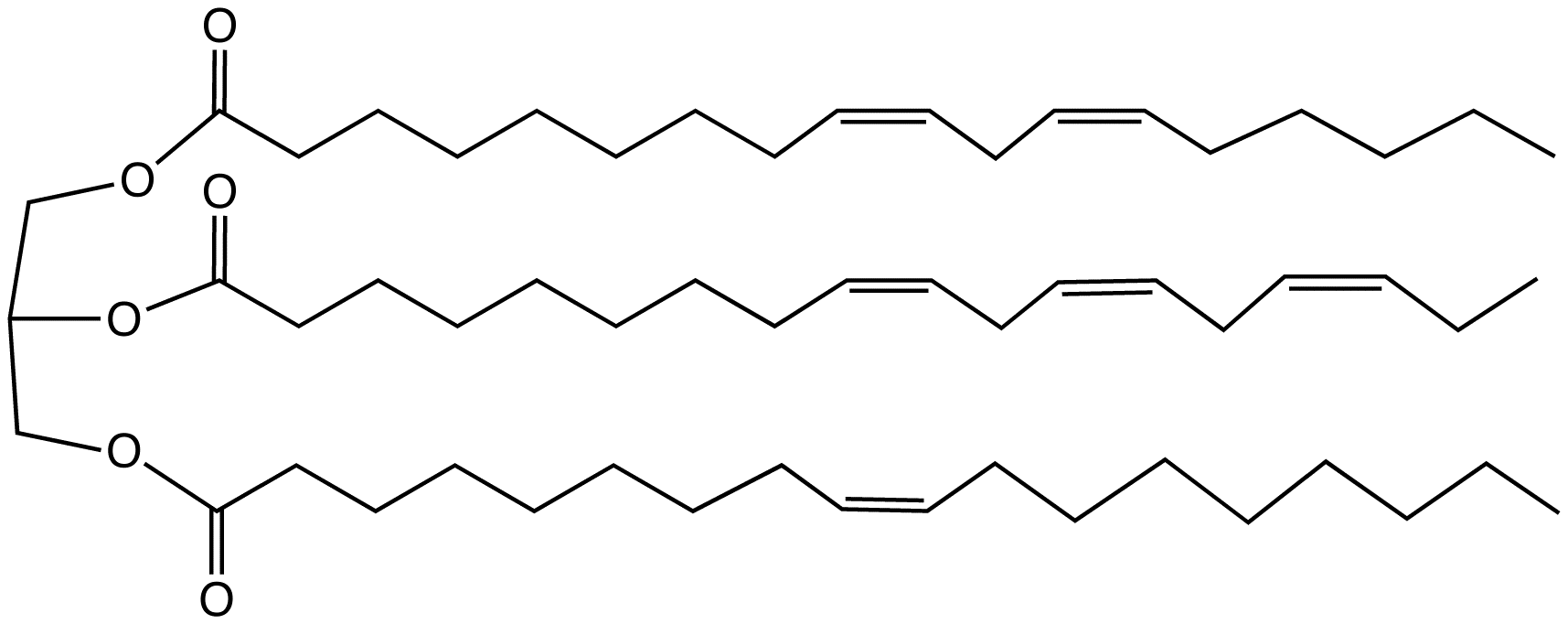
Triglyceride Unsaturated Structural Formulae V
A triglyceride (TG, triacylglycerol, TAG, or triacylglyceride) is an ester derived from glycerol and three fatty acids (from ''tri-'' and ''glyceride''). Triglycerides are the main constituents of body fat in humans and other vertebrates, as well as vegetable fat. They are also present in the blood to enable the bidirectional transference of adipose fat and blood glucose from the liver, and are a major component of human skin oils. Many types of triglycerides exist. One specific classification focuses on saturated and unsaturated types. Saturated fats have ''no'' C=C groups; unsaturated fats feature one or more C=C groups. Unsaturated fats tend to have a lower melting point than saturated analogues; as a result, they are often liquid at room temperature. Chemical structure Triglycerides are tri-esters consisting of a glycerol bound to three fatty acid molecules. Alcohols have a hydroxyl (HO–) group. Organic acids have a carboxyl (–COOH) group. Alcohols and organic ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Varnish
Varnish is a clear transparent hard protective coating or film. It is not a stain. It usually has a yellowish shade from the manufacturing process and materials used, but it may also be pigmented as desired, and is sold commercially in various shades. Varnish is primarily used as a wood finish where, stained or not, the distinctive tones and grains in the wood are intended to be visible. Varnish finishes are naturally glossy, but satin/semi-gloss and flat sheens are available. History The word "varnish" comes from Mediaeval Latin ''vernix'', meaning odorous resin, itself derived from Middle Greek ''berōnikón'' or ''beroníkē'', meaning amber or amber-colored glass. A false etymology traces the word to the Greek ''Berenice'', the ancient name of modern Benghazi in Libya, where the first varnishes in the Mediterranean area were supposedly used and where resins from the trees of now-vanished forests were sold. Early varnishes were developed by mixing resin—pine sap, for ex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oil Paint
Oil paint is a type of slow-drying paint that consists of particles of pigment suspended in a drying oil, commonly linseed oil. The viscosity of the paint may be modified by the addition of a solvent such as turpentine or white spirit, and varnish may be added to increase the glossiness of the dried oil paint film. The addition of oil or alkyd medium can also be used to modify the viscosity and drying time of oil paint. Oil paints were first used in Asia as early as the 7th century AD and can be seen in examples of Buddhist paintings in Afghanistan. Oil-based paints made their way to Europe by the 12th century and were used for simple decoration, but oil painting did not begin to be adopted as an artistic medium there until the early 15th century. Common modern applications of oil paint are in finishing and protection of wood in buildings and exposed metal structures such as ships and bridges. Its hard-wearing properties and luminous colors make it desirable for both interior ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drying Oil
A drying oil is an oil that hardens to a tough, solid film after a period of exposure to air, at room temperature. The oil hardens through a chemical reaction in which the components crosslink (and hence, polymerize) by the action of oxygen (not through the evaporation of water or other solvents). Drying oils are a key component of oil paint and some varnishes. Some commonly used drying oils include linseed oil, tung oil, poppy seed oil, perilla oil, and walnut oil. Their use has declined over the past several decades, as they have been replaced by alkyd resins and other binders. Since oxidation is the key to curing in these oils, those that are susceptible to chemical drying are often unsuitable for cooking, and are also highly susceptible to becoming rancid through autoxidation, the process by which fatty foods develop off-flavors. Rags, cloth, and paper saturated with drying oils may spontaneously combust (ignite) after a few hours as heat is released during the oxidation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Linoleic Acid
Linoleic acid (LA) is an organic compound with the formula COOH(CH2)7CH=CHCH2CH=CH(CH2)4CH3. Both alkene groups are cis-trans isomerism, ''cis''. It is a fatty acid sometimes denoted 18:2 (n-6) or 18:2 ''cis''-9,12. A linoleate is a salt (chemistry), salt or ester of this acid. Linoleic acid is a polyunsaturated fatty acid, polyunsaturated omega-6 fatty acid. It is a colorless liquid that is virtually insoluble in water but soluble in many organic solvents. It typically occurs in nature as a triglyceride (ester of glycerol, glycerin) rather than as a free fatty acid. It is one of two essential fatty acids for humans, who must obtain it through their diet, and the most essential, because the body uses it as a base to make the others. The word "linoleic" derives from the Latin ''linum'' "flax" + ''oleum'' "oil", reflecting the fact that it was first isolated from linseed oil. History In 1844, F. Sacc, working at the laboratory of Justus von Liebig, isolated linoleic acid from l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentadienyl
In organic chemistry, pentadienyl refers to the organic radical, anion, or cation with the formula , where ''z'' = 0, −1, +1, respectively. Organometallic chemistry In organometallic chemistry, the pentadienyl anion is a ligand, the acyclic analogue of the more-common cyclopentadienyl anion. The pentadienyl anion is generated by deprotonation of pentadiene. A number of complexes are known, including bis(pentadienyl) iron, , the "open" analog of ferrocene. Only few pentadienyl complexes feature simple ligands. More common is the dimethyl derivative 2,4-. Additionally, many pentadienyl ligands are cyclic, being derived from the addition of hydride to ''η''6-arene complexes or hydride abstraction from cyclohexadiene complexes. The first pentadienyl complex to be reported was derived from protonolysis of a complex of pentadienol: :Fe(C5H7OH)(CO)3 + H+ -> e(C5H7)(CO)3 + H2O Treatment of this cation with sodium borohydride gives the pentadiene complex: : e(C5H7)(CO)3 + H- -> Fe(C5H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Dissociation Energy
The bond-dissociation energy (BDE, ''D''0, or ''DH°'') is one measure of the strength of a chemical bond . It can be defined as the standard enthalpy change when is cleaved by homolysis to give fragments A and B, which are usually radical species. The enthalpy change is temperature-dependent, and the bond-dissociation energy is often defined to be the enthalpy change of the homolysis at 0 K (absolute zero), although the enthalpy change at 298 K (standard conditions) is also a frequently encountered parameter. As a typical example, the bond-dissociation energy for one of the C−H bonds in ethane () is defined as the standard enthalpy change of the process : , : ''DH''°298() = Δ''H°'' = 101.1(4) kcal/mol = 423.0 ± 1.7 kJ/mol = 4.40(2) eV (per bond). To convert a molar BDE to the energy needed to dissociate the bond ''per molecule'', the conversion factor 23.060 kcal/mol (96.485 kJ/mol) for each eV can be used. A variety of experim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |