Hydrogenation on:
[Wikipedia]
[Google]
[Amazon]
 Hydrogenation is a
Hydrogenation is a
File:Dichlorotris(triphenylphosphine)ruthenium(II).png,
 Homogeneous catalysts are also used in asymmetric synthesis by the hydrogenation of prochiral substrates. An early demonstration of this approach was the Rh-catalyzed hydrogenation of enamides as precursors to the drug L-DOPA. To achieve asymmetric reduction, these catalyst are made chiral by use of chiral diphosphine ligands. Rhodium catalyzed hydrogenation has also been used in the herbicide production of S-metolachlor, which uses a Josiphos type ligand (called Xyliphos). In principle asymmetric hydrogenation can be catalyzed by chiral heterogeneous catalysts, but this approach remains more of a curiosity than a useful technology.
Homogeneous catalysts are also used in asymmetric synthesis by the hydrogenation of prochiral substrates. An early demonstration of this approach was the Rh-catalyzed hydrogenation of enamides as precursors to the drug L-DOPA. To achieve asymmetric reduction, these catalyst are made chiral by use of chiral diphosphine ligands. Rhodium catalyzed hydrogenation has also been used in the herbicide production of S-metolachlor, which uses a Josiphos type ligand (called Xyliphos). In principle asymmetric hydrogenation can be catalyzed by chiral heterogeneous catalysts, but this approach remains more of a curiosity than a useful technology.
File:CarvoneH2.png, Selective hydrogenation of the less hindered alkene group in
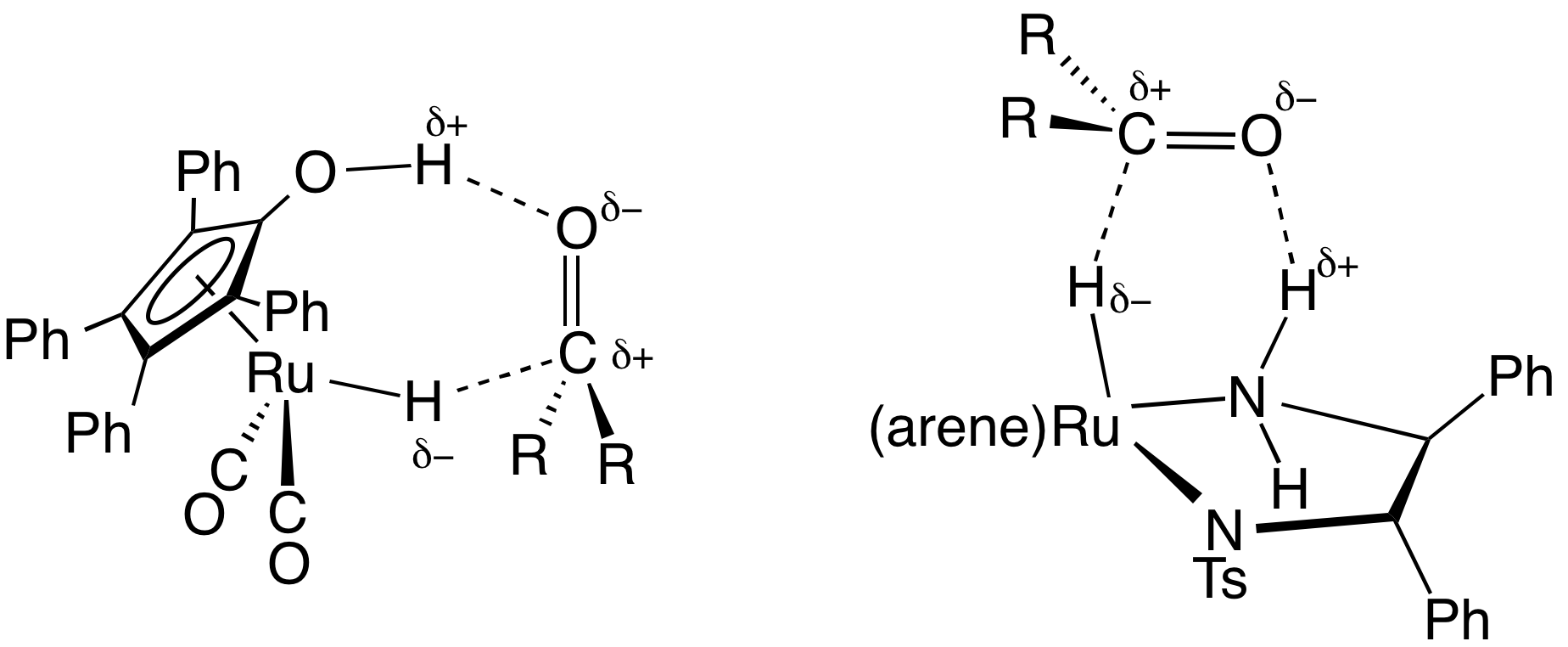 ''Transfer hydrogenation'' uses hydrogen-donor molecules other than molecular H2. These "sacrificial" hydrogen donors, which can also serve as
''Transfer hydrogenation'' uses hydrogen-donor molecules other than molecular H2. These "sacrificial" hydrogen donors, which can also serve as
RCH=CH2 + D2 -> RCHDCH2D
L_\mathitM + H2 -> L_\mathitMH2
* binding of alkene:
*:L_\mathitM(\eta^2 H2) + CH2=CHR -> L_MH2(CH2=CHR) + L
* transfer of one hydrogen atom from the metal to carbon (migratory insertion)
*:L_MH2(CH2=CHR) -> L_M(H)(CH2-CH2R)
* transfer of the second hydrogen atom from the metal to the alkyl group with simultaneous dissociation of the alkane ("reductive elimination")
*:L_M(H)(CH2-CH2R) -> L_M + CH3-CH2R
\underset + \underset -> ce350-550^\circ\ce C] \underset
Oxygen can be partially hydrogenated to give
 A
A  The reduction of
The reduction of
high pressure hydrogen generators
which generate hydrogen up to 100 bar (1400 PSI) from water. Heat may also be used, as the pressure compensates for the associated reduction in gas solubility.
Organic Syntheses, Coll. Vol. 7, p.226 (1990).
*
Organic Syntheses, Coll. Vol. 8, p.609 (1993).
*
Organic Syntheses, Coll. Vol. 5, p.552 (1973).
*
Organic Syntheses, Coll. Vol. 3, p.720 (1955).
*
Organic Syntheses, Coll. Vol. 6, p.371 (1988).
* early work on transfer hydrogenation: ** ** **
"The Magic of Hydro"
''
chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
between molecular hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
(H2) and another compound or element, usually in the presence of a catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
such as nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to ...
, palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself na ...
or platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Platinu ...
. The process is commonly employed to reduce
Reduction, reduced, or reduce may refer to:
Science and technology Chemistry
* Reduction (chemistry), part of a reduction-oxidation (redox) reaction in which atoms have their oxidation state changed.
** Organic redox reaction, a redox react ...
or saturate
Saturate may refer to:
* ''Saturate'' (Breaking Benjamin album), 2002
* ''Saturate'' (Gojira album), 1999
* ''Saturate'' (Jeff Deyo album), 2002
* " Electronic Battle Weapon 8", a song by The Chemical Brothers, a shorter version of which was re ...
organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The ...
s. Hydrogenation typically constitutes the addition of pairs of hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
s to a molecule, often an alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
. Catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
s are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double
A double is a look-alike or doppelgänger; one person or being that resembles another.
Double, The Double or Dubble may also refer to:
Film and television
* Double (filmmaking), someone who substitutes for the credited actor of a character
* Th ...
and triple
Triple is used in several contexts to mean "threefold" or a " treble":
Sports
* Triple (baseball), a three-base hit
* A basketball three-point field goal
* A figure skating jump with three rotations
* In bowling terms, three strikes in a row
* ...
bonds in hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ex ...
s.
Process
Hydrogenation has three components, the unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst.Related or competing reactions
The same catalysts and conditions that are used for hydrogenation reactions can also lead toisomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomeriz ...
of the alkenes
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
from cis to trans. This process is of great interest because hydrogenation technology generates most of the trans fat
Trans fat, also called trans-unsaturated fatty acids, or trans fatty acids, is a type of unsaturated fat that naturally occurs in small amounts in meat and milk fat. It became widely produced as an unintentional byproduct in the industrial pro ...
in foods (see below). A reaction where bonds are broken while hydrogen is added is called hydrogenolysis
Hydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes lysis (breakdown) by hydrogen.Ralph Connor, Homer Adkins. Hydrogenolysis Of Oxygenated Organic Compounds. J. Am. Chem. Soc. ...
, a reaction that may occur to carbon-carbon and carbon-heteroatom (oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
, nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
or halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
) bonds. Some hydrogenations of polar bonds are accompanied by hydrogenolysis.
Hydrogen sources
For hydrogenation, the obvious source of hydrogen is H2 gas itself, which is typically available commercially within the storage medium of a pressurized cylinder. The hydrogenation process often uses greater than 1 atmosphere of H2, usually conveyed from the cylinders and sometimes augmented by "booster pumps". Gaseous hydrogen is produced industrially from hydrocarbons by the process known assteam reforming
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly natural gas is the feedstock. The main purpose of this technology is hydrogen product ...
.Paul N. Rylander, "Hydrogenation and Dehydrogenation" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2005. For many applications, hydrogen is transferred from donor molecules such as formic acid, isopropanol, and dihydroanthracene
9,10-Dihydroanthracene is an organic compound that is derived from the polycyclic aromatic hydrocarbon anthracene. Several isomers of dihydroanthracene are known, but the 9,10 derivative is most common. It is a colourless solid that is used as a ...
. These hydrogen donors undergo dehydrogenation
In chemistry, dehydrogenation is a chemical reaction that involves the removal of hydrogen, usually from an organic molecule. It is the reverse of hydrogenation. Dehydrogenation is important, both as a useful reaction and a serious problem. At ...
to, respectively, carbon dioxide, acetone, and anthracene
Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the Economic production, production of the red dye alizarin and other dyes ...
. These processes are called transfer hydrogenation
In chemistry, transfer hydrogenation is a chemical reaction involving the addition of hydrogen to a compound from a source other than molecular . It is applied in laboratory and industrial organic synthesis to saturate organic compounds and redu ...
s.
Substrates
An important characteristic of alkene and alkyne hydrogenations, both the homogeneously and heterogeneously catalyzed versions, is that hydrogen addition occurs with " syn addition", with hydrogen entering from the least hindered side. This reaction can be performed on a variety of differentfunctional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
s.
Catalysts
With rare exceptions, H2 is unreactive toward organic compounds in the absence of metal catalysts. The unsaturated substrate is chemisorbed onto the catalyst, with most sites covered by the substrate. In heterogeneous catalysts, hydrogen forms surface hydrides (M-H) from which hydrogens can be transferred to the chemisorbed substrate.Platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Platinu ...
, palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself na ...
, rhodium
Rhodium is a chemical element with the symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring isoto ...
, and ruthenium
Ruthenium is a chemical element with the Symbol (chemistry), symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to ...
form highly active catalysts, which operate at lower temperatures and lower pressures of H2. Non-precious metal catalysts, especially those based on nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to ...
(such as Raney nickel and Urushibara nickel Urushibara nickel is a nickel based hydrogenation catalyst, named after Yoshiyuki Urushibara.
History
It was discovered by Yoshiyuki Urushibara in 1951, while doing research on the reduction of estrone to estradiol.
Preparation
First nickel is pr ...
) have also been developed as economical alternatives, but they are often slower or require higher temperatures. The trade-off is activity (speed of reaction) vs. cost of the catalyst and cost of the apparatus required for use of high pressures. Notice that the Raney-nickel catalysed hydrogenations require high pressures:
Catalysts are usually classified into two broad classes: homogeneous catalyst
In chemistry, homogeneous catalysis is catalysis by a soluble catalyst in a solution. Homogeneous catalysis refers to reactions where the catalyst is in the same phase as the reactants, principally in solution. In contrast, heterogeneous catalysis ...
s and heterogeneous catalyst
In chemistry, heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reactants or products. The process contrasts with homogeneous catalysis where the reactants, products and catalyst exist in the same phase. P ...
s. Homogeneous catalysts dissolve in the solvent that contains the unsaturated substrate. Heterogeneous catalysts are solids that are suspended in the same solvent with the substrate or are treated with gaseous substrate.
Homogeneous catalysts
Some well known homogeneous catalysts are indicated below. These arecoordination complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
es that activate both the unsaturated substrate and the H2. Most typically, these complexes contain platinum group metals, especially Rh and Ir.
Dichlorotris(triphenylphosphine)ruthenium(II)
Dichlorotris(triphenylphosphine)ruthenium(II) is a coordination complex of ruthenium. It is a chocolate brown solid that is soluble in organic solvents such as benzene. The compound is used as a precursor to other complexes including those used i ...
is a precatalyst based on ruthenium.
File:Crabtree.svg, Crabtree's catalyst
Crabtree's catalyst is an organoiridium compound with the formula ,5-Cyclooctadiene, C8H12IrTricyclohexylphosphine, P(C6H11)3pyridine, C5H5NF6. It is a homogeneous catalyst for hydrogenation and hydrogen-transfer reactions, developed by Robert ...
is a highly active catalyst featuring iridium.
File:Cyclooctadiene-rhodium-chloride-dimer-2D-skeletal.png, Rh2Cl2(cod)2 is a precursor to many homogeneous catalysts.
File:(S)-iPr-PHOX.svg, (S)-iPr-PHOX
(''S'')-iPr-PHOX, or (''S'')-2- -(diphenylphosphino)phenyl4-isopropyl-4,5-dihydrooxazole, is a chiral, bidentate, ligand derived from the amino alcohol valinol. It is part of a broader class of phosphinooxazolines ligands and has found applicat ...
is a typical chelating phosphine ligand used in asymmetric hydrogenation.
 Homogeneous catalysts are also used in asymmetric synthesis by the hydrogenation of prochiral substrates. An early demonstration of this approach was the Rh-catalyzed hydrogenation of enamides as precursors to the drug L-DOPA. To achieve asymmetric reduction, these catalyst are made chiral by use of chiral diphosphine ligands. Rhodium catalyzed hydrogenation has also been used in the herbicide production of S-metolachlor, which uses a Josiphos type ligand (called Xyliphos). In principle asymmetric hydrogenation can be catalyzed by chiral heterogeneous catalysts, but this approach remains more of a curiosity than a useful technology.
Homogeneous catalysts are also used in asymmetric synthesis by the hydrogenation of prochiral substrates. An early demonstration of this approach was the Rh-catalyzed hydrogenation of enamides as precursors to the drug L-DOPA. To achieve asymmetric reduction, these catalyst are made chiral by use of chiral diphosphine ligands. Rhodium catalyzed hydrogenation has also been used in the herbicide production of S-metolachlor, which uses a Josiphos type ligand (called Xyliphos). In principle asymmetric hydrogenation can be catalyzed by chiral heterogeneous catalysts, but this approach remains more of a curiosity than a useful technology.
Heterogeneous catalysts
Heterogeneous catalysts for hydrogenation are more common industrially. In industry, precious metal hydrogenation catalysts are deposited from solution as a fine powder on the support, which is a cheap, bulky, porous, usually granular material, such asactivated carbon
Activated carbon, also called activated charcoal, is a form of carbon commonly used to filter contaminants from water and air, among many other uses. It is processed (activated) to have small, low-volume pores that increase the surface area avail ...
, alumina, calcium carbonate or barium sulfate
Barium sulfate (or sulphate) is the inorganic compound with the chemical formula Ba SO4. It is a white crystalline solid that is odorless and insoluble in water. It occurs as the mineral barite, which is the main commercial source of barium an ...
. For example, platinum on carbon is produced by reduction of chloroplatinic acid
Chloroplatinic acid (also known as hexachloroplatinic acid) is an inorganic compound with the formula 3Osub>2 tCl6H2O)''x'' (0 ≤ ''x'' ≤ 6). A red solid, it is an important commercial source of platinum, usually as an aqueous solution. Alth ...
''in situ'' in carbon. Examples of these catalysts are 5% ruthenium
Ruthenium is a chemical element with the Symbol (chemistry), symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to ...
on activated carbon
Activated carbon, also called activated charcoal, is a form of carbon commonly used to filter contaminants from water and air, among many other uses. It is processed (activated) to have small, low-volume pores that increase the surface area avail ...
, or 1% platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Platinu ...
on alumina. Base metal catalysts, such as Raney nickel, are typically much cheaper and do not need a support. Also, in the laboratory, unsupported (massive) precious metal catalysts such as platinum black
Platinum black (Pt black) is a fine powder of platinum with good catalytic properties. The name of platinum black is due to its black color. It is used in many ways; as a thin film electrode, a fuel cell membrane catalyst, or as a catalytic igniti ...
are still used, despite the cost.
As in homogeneous catalysts, the activity is adjusted through changes in the environment around the metal, i.e. the coordination sphere
In coordination chemistry, the first coordination sphere refers to the array of molecules and ions (the ligands) directly attached to the central metal atom. The second coordination sphere consists of molecules and ions that attached in various ...
. Different faces
The face is the front of an animal's head that features the eyes, nose and mouth, and through which animals express many of their emotions. The face is crucial for human identity, and damage such as scarring or developmental deformities may affe ...
of a crystalline heterogeneous catalyst display distinct activities, for example. This can be modified by mixing metals or using different preparation techniques. Similarly, heterogeneous catalysts are affected by their supports.
In many cases, highly empirical modifications involve selective "poisons". Thus, a carefully chosen catalyst can be used to hydrogenate some functional groups without affecting others, such as the hydrogenation of alkenes without touching aromatic rings, or the selective hydrogenation of alkynes
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no ...
to alkenes using Lindlar's catalyst. For example, when the catalyst palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself na ...
is placed on barium sulfate
Barium sulfate (or sulphate) is the inorganic compound with the chemical formula Ba SO4. It is a white crystalline solid that is odorless and insoluble in water. It occurs as the mineral barite, which is the main commercial source of barium an ...
and then treated with quinoline
Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only sli ...
, the resulting catalyst reduces alkynes only as far as alkenes. The Lindlar catalyst has been applied to the conversion of phenylacetylene
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas.
Preparation
I ...
to styrene
Styrene () is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concen ...
.
carvone
Carvone is a member of a family of chemicals called terpenoids. Carvone is found naturally in many essential oils, but is most abundant in the oils from seeds of caraway (''Carum carvi''), spearmint (''Mentha spicata''), and dill.
Uses
Both c ...
using a homogeneous catalyst (Wilkinson's catalyst
Wilkinson's catalyst is the common name for chloridotris(triphenylphosphine)rhodium(I), a coordination complex of rhodium with the formula hCl(PPh3)3(Ph = phenyl). It is a red-brown colored solid that is soluble in hydrocarbon solvents such as ...
).
File:PhC2HH2.png, Partial hydrogenation of phenylacetylene
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas.
Preparation
I ...
using the Lindlar catalyst
A Lindlar catalyst is a heterogeneous catalyst that consists of palladium deposited on calcium carbonate or barium sulfate which is then poisoned with various forms of lead or sulfur. It is used for the hydrogenation of alkynes to alkenes (i.e. ...
.
File:ImineH2.png, Hydrogenation of an imine using a Raney nickel catalyst, a popular heterogeneous catalyst.
File:ResorcinolH2.png, Partial hydrogenation of a resorcinol
Resorcinol (or resorcin) is an organic compound with the formula C6H4(OH)2. It is one of three isomeric benzenediols, the 1,3-isomer (or '' meta''-isomer). Resorcinol crystallizes from benzene as colorless needles that are readily soluble in w ...
derivative using a Raney-Nickel catalyst.
File:SuccPdH2.png, Hydrogenation of maleic acid
Maleic acid or ''cis''-butenedioic acid is an organic compound that is a dicarboxylic acid, a molecule with two carboxyl groups. Its chemical formula is HO2CCH=CHCO2H. Maleic acid is the ''cis''-isomer of butenedioic acid, whereas fumaric ac ...
to succinic acid
Succinic acid () is a dicarboxylic acid with the chemical formula (CH2)2(CO2H)2. The name derives from Latin ''succinum'', meaning amber. In living organisms, succinic acid takes the form of an anion, succinate, which has multiple biological ro ...
.
Transfer hydrogenation
 ''Transfer hydrogenation'' uses hydrogen-donor molecules other than molecular H2. These "sacrificial" hydrogen donors, which can also serve as
''Transfer hydrogenation'' uses hydrogen-donor molecules other than molecular H2. These "sacrificial" hydrogen donors, which can also serve as solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
s for the reaction, include hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine ...
, formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid, and has the chemical formula HCOOH and structure . It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Es ...
, and alcohols such as isopropanol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group (chemical formula ) it is the simple ...
.
In organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
, transfer hydrogenation is useful for the asymmetric hydrogenation
Asymmetric hydrogenation is a chemical reaction that adds two atoms of hydrogen to a target (substrate) molecule with three-dimensional spatial selectivity. Critically, this selectivity does not come from the target molecule itself, but from othe ...
of polar unsaturated substrates, such as ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
s, aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
s and imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bo ...
s, by employing chiral catalysts.
Electrolytic hydrogenation
Polar
Polar may refer to:
Geography
Polar may refer to:
* Geographical pole, either of two fixed points on the surface of a rotating body or planet, at 90 degrees from the equator, based on the axis around which a body rotates
* Polar climate, the c ...
substrates such as nitriles
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix ''cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including met ...
can be hydrogenated electrochemically, using protic solvent
In chemistry, a protic solvent is a solvent that has a hydrogen atom bound to an oxygen (as in a hydroxyl group ), a nitrogen (as in an amine group or ), or fluoride (as in hydrogen fluoride). In general terms, any solvent that contains a Labile# ...
s and reducing equivalents as the source of hydrogen.
Thermodynamics and mechanism
The addition of hydrogen to double or triple bonds inhydrocarbons
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ex ...
is a type of redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction ...
reaction that can be thermodynamically favorable. For example, the addition of hydrogen to ethene has a Gibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and pr ...
change of -101 kJ·mol−1, which is highly exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e ...
. In the hydrogenation of vegetable oils and fatty acids, for example, the heat released, about 25 kcal per mole (105 kJ/mol), is sufficient to raise the temperature of the oil by 1.6–1.7 °C per iodine number
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a vio ...
drop.
However, the reaction rate for most hydrogenation reactions is negligible in the absence of catalysts. The mechanism
Mechanism may refer to:
* Mechanism (engineering), rigid bodies connected by joints in order to accomplish a desired force and/or motion transmission
*Mechanism (biology), explaining how a feature is created
*Mechanism (philosophy), a theory that ...
of metal-catalyzed hydrogenation of alkenes and alkynes has been extensively studied. First of all isotope labeling
Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope (an atom with a detectable variation in neutron count) through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific ...
using deuterium
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two Stable isotope ratio, stable isotopes of hydrogen (the other being Hydrogen atom, protium, or hydrogen-1). The atomic nucleus, nucleus of a deuterium ato ...
confirms the regiochemistry
In chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base ...
of the addition:
:Heterogeneous catalysis
On solids, the accepted mechanism is the Horiuti- Polanyi mechanism: # Binding of the unsaturated bond # Dissociation of on the catalyst # Addition of one atom of hydrogen; this step is reversible # Addition of the second atom; effectively irreversible. In the third step, the alkyl group can revert to alkene, which can detach from the catalyst. Consequently, contact with a hydrogenation catalyst allows ''cis-trans''-isomerization. The ''trans''-alkene can reassociate to the surface and undergo hydrogenation. These details are revealed in part using D2 (deuterium), because recovered alkenes often contain deuterium. For aromatic substrates, the first hydrogenation is slowest. The product of this step is a cyclohexadiene, which hydrogenate rapidly and are rarely detected. Similarly, thecyclohexene
Cyclohexene is a hydrocarbon with the formula C6H10. This cycloalkene is a colorless liquid with a sharp smell. It is an intermediate in various industrial processes. Cyclohexene is not very stable upon long term storage with exposure to light a ...
is ordinarily reduced to cyclohexane.
Homogeneous catalysis
In many homogeneous hydrogenation processes, the metal binds to both components to give an intermediate alkene-metal(H)2 complex. The general sequence of reactions is assumed to be as follows or a related sequence of steps: * binding of the hydrogen to give a dihydride complex viaoxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidat ...
(preceding the oxidative addition of is the formation of a dihydrogen complex
Dihydrogen complexes are coordination complexes containing intact H2 as a ligand. They are a subset of sigma complexes. The prototypical complex is W(CO)3( PCy3)2(H2). This class of compounds represent intermediates in metal-catalyzed reactions ...
):
*:Inorganic substrates
The hydrogenation of nitrogen to give ammonia is conducted on a vast scale by theHaber–Bosch
The Haber process, also called the Haber–Bosch process, is an artificial nitrogen fixation process and is the main industrial procedure for the production of ammonia today. It is named after its inventors, the German chemists Fritz Haber and C ...
process, consuming an estimated 1% of the world's energy supply.
:hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%� ...
, although this process has not been commercialized. One difficulty is preventing the catalysts from triggering decomposition of the hydrogen peroxide to form water.
Industrial applications
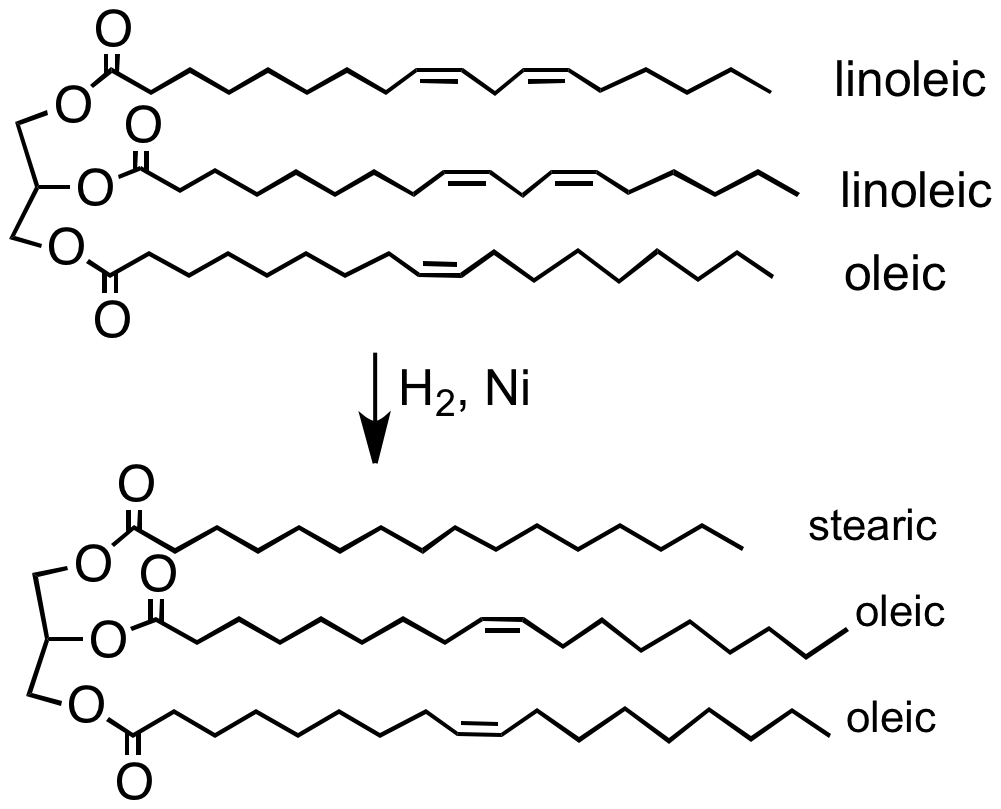
Catalytic hydrogenation has diverse industrial uses. Most frequently, industrial hydrogenation relies on heterogeneous catalysts.Food industry
The food industry hydrogenates vegetable oils to convert them into solid or semi-solid fats that can be used in spreads, candies, baked goods, and other products likemargarine
Margarine (, also , ) is a spread used for flavoring, baking, and cooking. It is most often used as a substitute for butter. Although originally made from animal fats, most margarine consumed today is made from vegetable oil. The spread was orig ...
. Vegetable oils are made from polyunsaturated fatty acids (having more than one carbon-carbon double bond). Hydrogenation eliminates some of these double bonds.Ian P. Freeman "Margarines and Shortenings" in ''Ullmann's Encyclopedia of Industrial Chemistry'', 2005, Wiley-VCH, Weinheim.
:
Petrochemical industry
In petrochemical processes, hydrogenation is used to convert alkenes and aromatics into saturated alkanes (paraffins) and cycloalkanes (naphthenes), which are less toxic and less reactive. Relevant to liquid fuels that are stored sometimes for long periods in air, saturated hydrocarbons exhibit superior storage properties. On the other hand, alkenes tend to formhydroperoxide
Hydroperoxides or peroxols are compounds containing the hydroperoxide functional group (ROOH). If the R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have the formula ROOR. ...
s, which can form gums that interfere with fuel handling equipment. For example, mineral turpentine
White spirit (AU, UK & Ireland)Primarily in the United Kingdom and Australia. In New Zealand "white spirit" can also refer to Coleman fuel (white gas). or mineral spirits (US, Canada), also known as mineral turpentine (AU/NZ), turpentine substit ...
is usually hydrogenated. Hydrocracking
In petrochemistry, petroleum geology and organic chemistry, cracking is the process whereby complex organic molecules such as kerogens or long-chain hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking of ...
of heavy residues into diesel is another application. In isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomeriz ...
and catalytic reforming
Catalytic reforming is a chemical process used to convert petroleum refinery naphthas distilled from crude oil (typically having low octane ratings) into high-octane liquid products called reformates, which are premium blending stocks for high-oc ...
processes, some hydrogen pressure is maintained to hydrogenolyze coke formed on the catalyst and prevent its accumulation.
Organic chemistry
Hydrogenation is a useful means for converting unsaturated compounds into saturated derivatives. Substrates include not only alkenes and alkynes, but also aldehydes, imines, and nitriles, which are converted into the corresponding saturated compounds, i.e. alcohols and amines. Thus, alkyl aldehydes, which can be synthesized with theoxo process
Hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon d ...
from carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
and an alkene, can be converted to alcohols. E.g. 1-propanol is produced from propionaldehyde, produced from ethene and carbon monoxide. Xylitol
Xylitol is a chemical compound with the formula , or HO(CH2)(CHOH)3(CH2)OH; specifically, one particular stereoisomer with that structural formula. It is a colorless or white crystalline solid that is freely soluble in water. It can be classifie ...
, a polyol
In organic chemistry, a polyol is an organic compound containing multiple hydroxyl groups (). The term "polyol" can have slightly different meanings depending on whether it is used in food science or polymer chemistry. Polyols containing two, thr ...
, is produced by hydrogenation of the sugar xylose
Xylose ( grc, ξύλον, , "wood") is a sugar first isolated from wood, and named for it. Xylose is classified as a monosaccharide of the aldopentose type, which means that it contains five carbon atoms and includes an aldehyde functional gro ...
, an aldehyde. Primary amines can be synthesized by hydrogenation of nitriles, while nitriles are readily synthesized from cyanide and a suitable electrophile. For example, isophorone diamine, a precursor to the polyurethane
Polyurethane (; often abbreviated PUR and PU) refers to a class of polymers composed of organic chemistry, organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethan ...
monomer isophorone diisocyanate
Isophorone diisocyanate (IPDI) is an organic compound in the class known as isocyanates. More specifically, it is an aliphatic diisocyanate. It is produced in relatively small quantities, accounting for (with hexamethylene diisocyanate) only 3.4% ...
, is produced from isophorone nitrile by a tandem nitrile hydrogenation/reductive amination by ammonia, wherein hydrogenation converts both the nitrile into an amine and the imine formed from the aldehyde and ammonia into another amine.
Hydrogenation of coal
History
Heterogeneous catalytic hydrogenation
The earliest hydrogenation is that ofplatinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Platinu ...
catalyzed
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
addition of hydrogen to oxygen in the Döbereiner's lamp
Döbereiner's lamp, also called a "tinderbox" ("Feuerzeug"), is a lighter invented in 1823 by the German chemist Johann Wolfgang Döbereiner. The lighter is based on the Fürstenberger lighter (invented in Basel in 1780; in which hydrogen gas is ...
, a device commercialized as early as 1823. The French chemist Paul Sabatier Paul Sabatier may refer to:
*Paul Sabatier (chemist) (1854–1941), French chemist and Nobel Prize winner
*Paul Sabatier (theologian)
Charles Paul Marie Sabatier (3 or 9 August 1858 – 5 March 1928), was a French clergyman and historian who prod ...
is considered the father of the hydrogenation process. In 1897, building on the earlier work of James Boyce, an American chemist working in the manufacture of soap products, he discovered that traces of nickel catalyzed the addition of hydrogen to molecules of gaseous hydrocarbons in what is now known as the Sabatier process. For this work, Sabatier shared the 1912 Nobel Prize in Chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
. Wilhelm Normann
Wilhelm Normann (16 January 1870, in Petershagen – 1 May 1939, in Chemnitz) (sometimes also spelled ''Norman'') was a German chemist who introduced the hydrogenation of fats in 1901, creating what later became known as trans fats. This inventio ...
was awarded a patent in Germany in 1902 and in Britain in 1903 for the hydrogenation of liquid oils, which was the beginning of what is now a worldwide industry. The commercially important Haber–Bosch process
The Haber process, also called the Haber–Bosch process, is an artificial nitrogen fixation process and is the main industrial procedure for the production of ammonia today. It is named after its inventors, the German chemists Fritz Haber and C ...
, first described in 1905, involves hydrogenation of nitrogen. In the Fischer–Tropsch process
The Fischer–Tropsch process is a collection of chemical reactions that converts a mixture of carbon monoxide and hydrogen, known as syngas, into liquid hydrocarbons. These reactions occur in the presence of metal catalysts, typically at temperat ...
, reported in 1922 carbon monoxide, which is easily derived from coal, is hydrogenated to liquid fuels.
In 1922, Voorhees and Adams described an apparatus for performing hydrogenation under pressures above one atmosphere. The Parr shaker, the first product to allow hydrogenation using elevated pressures and temperatures, was commercialized in 1926 based on Voorhees and Adams' research and remains in widespread use. In 1924 Murray Raney developed a finely powdered form of nickel, which is widely used to catalyze hydrogenation reactions such as conversion of nitriles to amines or the production of margarine.
Homogeneous catalytic hydrogenation
In the 1930s, Calvin discovered that copper(II) complexes oxidized H2. The 1960s witnessed the development of well definedhomogeneous catalyst
In chemistry, homogeneous catalysis is catalysis by a soluble catalyst in a solution. Homogeneous catalysis refers to reactions where the catalyst is in the same phase as the reactants, principally in solution. In contrast, heterogeneous catalysis ...
s using transition metal complexes, e.g., Wilkinson's catalyst
Wilkinson's catalyst is the common name for chloridotris(triphenylphosphine)rhodium(I), a coordination complex of rhodium with the formula hCl(PPh3)3(Ph = phenyl). It is a red-brown colored solid that is soluble in hydrocarbon solvents such as ...
(RhCl(PPh3)3). Soon thereafter cationic Rh and Ir were found to catalyze the hydrogenation of alkenes and carbonyls. In the 1970s, asymmetric hydrogenation was demonstrated in the synthesis of L-DOPA
-DOPA, also known as levodopa and -3,4-dihydroxyphenylalanine, is an amino acid that is made and used as part of the normal biology of some plants and animals, including humans. Humans, as well as a portion of the other animals that utilize -DOPA ...
, and the 1990s saw the invention of Noyori asymmetric hydrogenation
In chemistry, the Noyori asymmetric hydrogenation refers to methodology for enantioselective reduction of ketones and related functional groups. This methodology was introduced by Ryoji Noyori, who shared the Nobel Prize in Chemistry in 2001 for c ...
. The development of homogeneous hydrogenation was influenced by work started in the 1930s and 1940s on the oxo process
Hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon d ...
and Ziegler–Natta polymerization.
Metal-free hydrogenation
For most practical purposes, hydrogenation requires a metal catalyst. Hydrogenation can, however, proceed from some hydrogen donors without catalysts, illustrative hydrogen donors beingdiimide
Diimide, also called diazene or diimine, is a compound having the formula (NH)2. It exists as two geometric isomers, ''E'' (''trans'') and ''Z'' (''cis''). The term diazene is more common for organic derivatives of diimide. Thus, azobenzene is ...
and aluminium isopropoxide
Aluminium isopropoxide is the chemical compound usually described with the formula Al(O-''i''-Pr)3, where ''i''-Pr is the isopropyl group (–CH(CH3)2). This colourless solid is a useful reagent in organic synthesis.
Structure
A tetrameric st ...
, the latter illustrated by the Meerwein–Ponndorf–Verley reduction
The Meerwein–Ponndorf–Verley (MPV) reduction in organic chemistry is the reduction of ketones and aldehydes to their corresponding alcohols utilizing aluminium alkoxide catalysis in the presence of a sacrificial alcohol. The advantages of the ...
. Some metal-free catalytic systems have been investigated in academic research. One such system for reduction of ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
s consists of ''tert''-butanol and potassium tert-butoxide
Potassium ''tert''-butoxide is the chemical compound with the formula K+(CH3)3CO−. This colourless solid is a strong base (pKa of conjugate acid around 17), which is useful in organic synthesis. It exists as a tetrameric cubane-type cluster ...
and very high temperatures. The reaction depicted below describes the hydrogenation of benzophenone
Benzophenone is the organic compound with the formula (C6H5)2CO, generally abbreviated Ph2CO. It is a white solid that is soluble in organic solvents. Benzophenone is a widely used building block in organic chemistry, being the parent diarylket ...
:
:chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is to be contrasted with chemical thermodynamics, which deals with the direction in wh ...
study found this reaction is first-order
In mathematics and other formal sciences, first-order or first order most often means either:
* "linear" (a polynomial of degree at most one), as in first-order approximation and other calculus uses, where it is contrasted with "polynomials of high ...
in all three reactants suggesting a cyclic 6-membered transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked wi ...
.
Another system for metal-free hydrogenation is based on the phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
-borane
Trihydridoboron, also known as borane or borine, is an unstable and highly reactive molecule with the chemical formula . The preparation of borane carbonyl, BH3(CO), played an important role in exploring the chemistry of boranes, as it indicated ...
, compound 1, which has been called a ''frustrated Lewis pair
A frustrated Lewis pair (FLP) is a compound or mixture containing a Lewis acid and a Lewis base that, because of steric hindrance, cannot combine to form a classical adduct. Many kinds of FLPs have been devised, and many simple substrates exhibi ...
''. It reversibly accepts dihydrogen at relatively low temperatures to form the phosphonium
In polyatomic cations with the chemical formula (where R is a hydrogen or an alkyl, aryl, or halide group). These cations have tetrahedral structures. The salts are generally colorless or take the color of the anions.
Types of phosphonium ...
borate
A borate is any of several boron oxyanions, negative ions consisting of boron and oxygen, such as orthoborate , metaborate , or tetraborate ; or any salt with such anions, such as sodium metaborate, and disodium tetraborate . The name also refe ...
2 which can reduce simple hindered imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bo ...
s.
:nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5 NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor t ...
to aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine
In organic chemistry, an aromatic amine is an organic compound consisting of an aroma ...
has been reported to be catalysed by fullerene
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ...
, its mono-anion, atmospheric hydrogen and UV light.
Equipment used for hydrogenation
Today's bench chemist has three main choices of hydrogenation equipment: * Batch hydrogenation under atmospheric conditions * Batch hydrogenation at elevated temperature and/or pressure * Flow hydrogenationBatch hydrogenation under atmospheric conditions
The original and still a commonly practised form of hydrogenation in teaching laboratories, this process is usually effected by adding solid catalyst to around bottom flask
Round-bottom flasks (also called round-bottomed flasks or RB flasks) are types of Laboratory flask, flasks having spherical bottoms used as laboratory glassware, mostly for chemistry, chemical or biochemistry, biochemical work. They are typicall ...
of dissolved reactant which has been evacuated using nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
or argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
gas and sealing the mixture with a penetrable rubber seal. Hydrogen gas is then supplied from a H2-filled balloon
A balloon is a flexible bag that can be inflated with a gas, such as helium, hydrogen, nitrous oxide, oxygen, and air. For special tasks, balloons can be filled with smoke, liquid water, granular media (e.g. sand, flour or rice), or light so ...
. The resulting three phase mixture is agitated to promote mixing. Hydrogen uptake can be monitored, which can be useful for monitoring progress of a hydrogenation. This is achieved by either using a graduated tube containing a coloured liquid, usually aqueous copper sulfate Copper sulfate may refer to:
* Copper(II) sulfate, CuSO4, a common compound used as a fungicide and herbicide
* Copper(I) sulfate
Copper(I) sulfate, also known as cuprous sulfate, is an inorganic compound with the chemical formula Cu2 SO4. It ...
or with gauges for each reaction vessel.
Batch hydrogenation at elevated temperature and/or pressure
Since many hydrogenation reactions such ashydrogenolysis
Hydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes lysis (breakdown) by hydrogen.Ralph Connor, Homer Adkins. Hydrogenolysis Of Oxygenated Organic Compounds. J. Am. Chem. Soc. ...
of protecting groups
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
In man ...
and the reduction of aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
systems proceed extremely sluggishly at atmospheric temperature and pressure, pressurised systems are popular. In these cases, catalyst is added to a solution of reactant under an inert atmosphere in a pressure vessel
A pressure vessel is a container designed to hold gases or liquids at a pressure substantially different from the ambient pressure.
Construction methods and materials may be chosen to suit the pressure application, and will depend on the size o ...
. Hydrogen is added directly from a cylinder or built in laboratory hydrogen source, and the pressurized slurry is mechanically rocked to provide agitation, or a spinning basket is used. Recent advances in electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
technology have led to the development ohigh pressure hydrogen generators
which generate hydrogen up to 100 bar (1400 PSI) from water. Heat may also be used, as the pressure compensates for the associated reduction in gas solubility.
Flow hydrogenation
Flow hydrogenation has become a popular technique at the bench and increasingly the process scale. This technique involves continuously flowing a dilute stream of dissolved reactant over a fixed bed catalyst in the presence of hydrogen. Using establishedHPLC
High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify each component in a mixture. It relies on pumps to pa ...
technology, this technique allows the application of pressures from atmospheric to . Elevated temperatures may also be used. At the bench scale, systems use a range of pre-packed catalysts which eliminates the need for weighing and filtering pyrophoric
A substance is pyrophoric (from grc-gre, πυροφόρος, , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolith ...
catalysts.
Industrial reactors
Catalytic hydrogenation is done in a tubular plug-flow reactor (PFR) packed with a supported catalyst. The pressures and temperatures are typically high, although this depends on the catalyst. Catalyst loading is typically much lower than in laboratory batch hydrogenation, and various promoters are added to the metal, or mixed metals are used, to improve activity, selectivity and catalyst stability. The use of nickel is common despite its low activity, due to its low cost compared to precious metals. Gas Liquid Induction Reactors (Hydrogenator) are also used for carrying out catalytic hydrogenation.See also
*Carbon neutral fuel
Carbon-neutral fuel is fuel which produces no net-greenhouse gas emissions or carbon footprint. In practice, this usually means fuels that are made using carbon dioxide (CO2) as a feedstock. Proposed carbon-neutral fuels can broadly be grouped in ...
* Dehydrogenation
In chemistry, dehydrogenation is a chemical reaction that involves the removal of hydrogen, usually from an organic molecule. It is the reverse of hydrogenation. Dehydrogenation is important, both as a useful reaction and a serious problem. At ...
* H-Bio
* Hydrogenolysis
Hydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes lysis (breakdown) by hydrogen.Ralph Connor, Homer Adkins. Hydrogenolysis Of Oxygenated Organic Compounds. J. Am. Chem. Soc. ...
* Hydrodesulfurization
Hydrodesulfurization (HDS) is a catalytic chemical process widely used to remove sulfur (S) from natural gas and from refined petroleum products, such as gasoline or petrol, jet fuel, kerosene, diesel fuel, and fuel oils. The purpose of remov ...
, hydrotreater and oil desulfurization
* Josiphos ligands
A Josiphos ligand is a type of chiral diphosphine which has been modified to be substrate (chemistry), substrate-specific; they are widely used for enantioselective synthesis. -U. Blaser, W. Brieden, B. Pugin, F. Spindler, M. Studer and A. Togni ...
* Timeline of hydrogen technologies
This is a timeline of the history of hydrogen technology.
Timeline
16th century
* c. 1520 – First recorded observation of hydrogen by Paracelsus through dissolution of metals (iron, zinc, and tin) in sulfuric acid.
17th century
* 1625 – Fi ...
* Transfer hydrogenation
In chemistry, transfer hydrogenation is a chemical reaction involving the addition of hydrogen to a compound from a source other than molecular . It is applied in laboratory and industrial organic synthesis to saturate organic compounds and redu ...
* Rhodium-catalyzed hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic ...
* Trans fats
Trans fat, also called trans-unsaturated fatty acids, or trans fatty acids, is a type of unsaturated fat that naturally occurs in small amounts in meat and milk fat. It became widely produced as an unintentional byproduct in the industrial p ...
References
Further reading
* * examples of hydrogenation from Organic Syntheses: *Organic Syntheses, Coll. Vol. 7, p.226 (1990).
*
Organic Syntheses, Coll. Vol. 8, p.609 (1993).
*
Organic Syntheses, Coll. Vol. 5, p.552 (1973).
*
Organic Syntheses, Coll. Vol. 3, p.720 (1955).
*
Organic Syntheses, Coll. Vol. 6, p.371 (1988).
* early work on transfer hydrogenation: ** ** **
External links
"The Magic of Hydro"
''
Popular Mechanics
''Popular Mechanics'' (sometimes PM or PopMech) is a magazine of popular science and technology, featuring automotive, home, outdoor, electronics, science, do-it-yourself, and technology topics. Military topics, aviation and transportation o ...
'', June 1931, pp. 107–109 – early article for the general public on hydrogenation of oil produced in the 1930s
{{Authority control
Addition reactions
Homogeneous catalysis
Industrial processes
Hydrogen
Organic redox reactions
Oil refining
Oil shale technology
Synthetic fuel technologies