|
Aluminium Isopropoxide
Aluminium isopropoxide is the chemical compound usually described with the formula Al(O-''i''-Pr)3, where ''i''-Pr is the isopropyl group (–CH(CH3)2). This colourless solid is a useful reagent in organic synthesis. Structure A tetrameric structure of the crystalline material was verified by NMR spectroscopy and X-ray crystallography. The species is described by the formula Al[(μ-O-''i''-Pr)2Al(O-''i''-Pr)2]3. The unique central Al is octahedral, and three other Al centers adopt tetrahedral geometry. The idealised symmetry group, point group symmetry is ''D3''. Preparation This compound is commercially available. Industrially, it is prepared by the reaction between isopropyl alcohol and aluminium metal, or aluminium trichloride: :2 Al + 6 ''i''PrOH → 2 Al(O-''i''-Pr)3 +3H2 :AlCl3 + 3 ''i''PrOH → Al(O-''i''-Pr)3 + 3 HCl The procedure entails heating a mixture of aluminium, isopropyl alcohol, with a small amount of mercuric chloride. The process occurs via the formation o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopropanol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group (chemical formula ) it is the simplest example of a secondary alcohol, where the alcohol carbon atom is attached to two other carbon atoms. It is a structural isomer of propan-1-ol and ethyl methyl ether. It is used in the manufacture of a wide variety of industrial and household chemicals and is a common ingredient in products such as antiseptics, disinfectants, hand sanitizer and detergents. Well over one million tonnes is produced worldwide annually. Properties Isopropyl alcohol is miscible in water, ethanol, and chloroform as, like these compounds, isopropyl is a polar molecule. It dissolves ethyl cellulose, polyvinyl butyral, many oils, alkaloids, and natural resins. Unlike ethanol or methanol, isopropyl alcohol is not miscible with salt solutions and can be se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Amalgam
Aluminium can form an amalgam in solution with mercury. Aluminium amalgam may be prepared by either grinding aluminium pellets or wire in mercury, or by allowing aluminium wire to react with a solution of mercury(II) chloride in water. This amalgam is used as a chemical reagent to reduce compounds, such as the reduction of imines to amines. The aluminium is the ultimate electron donor, and the mercury serves to mediate the electron transfer. The reaction and the waste from it contains mercury, so special safety precautions and disposal methods are needed. As an environmentally friendlier alternative, hydrides or other reducing agents can often be used to accomplish the same synthetic result. An alloy of aluminium and gallium was proposed as a method of hydrogen generation, as the gallium renders the aluminium more reactive by preventing it from forming an oxide layer. Mercury has this same effect on aluminium, but also serves additional functions related to electron transfer tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vyacheslav Tishchenko
Vyacheslav Evgenievich Tishchenko (Вячеслав Евгеньевич Тищенко; 19 August 1861 – 25 February 1941) was a Russian chemist, best-known for the development of the Tishchenko reaction. Life and work Tishchenko was born in 1861 in St. Petersburg, where he attended school before undertaking studies at Saint Petersburg State University (which was named Saint Petersburg Imperial University at the time). He worked in the laboratory of Alexander Butlerov, studying the interaction of paraformaldehyde with hydrogen halide, hydrohalic acids. Tishchenko graduated in 1884 and worked with Dmitri Mendeleev as a laboratory assistant and lecture assistant. Tishchenko became a lecturer at St. Petersburg State University in 1891, where he taught analytical chemistry. He was sent to the World's Columbian Exposition, Chicago World's Fair in 1893 and the Exposition Universelle (1900), Paris Exposition in 1900 in order to report back to his home university on the chemical techn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Macromolecules (journal)
''Macromolecules'' is a peer-reviewed scientific journal that has been published since 1968 by the American Chemical Society. Initially published bimonthly, it became monthly in 1983 and then, in 1990, biweekly. ''Macromolecules'' is abstracted and indexed in Scopus, EBSCOhost, PubMed, Web of Science, and SwetsWise. The editor-in-chief An editor-in-chief (EIC), also known as lead editor or chief editor, is a publication's editorial leader who has final responsibility for its operations and policies. The highest-ranking editor of a publication may also be titled editor, managing ... is Marc A. Hillmyer. Its first editor was Dr. Field H. Winslow. References External links * {{DEFAULTSORT:Macromolecules (Journal) American Chemical Society academic journals Bimonthly journals English-language journals Publications established in 1968 Polymer chemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclic Ester
Lactones are cyclic carboxylic esters, containing a 1-oxacycloalkan-2-one structure (), or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring. Lactones are formed by intramolecular esterification of the corresponding hydroxycarboxylic acids, which takes place spontaneously when the ring that is formed is five- or six-membered. Lactones with three- or four-membered rings (α-lactones and β-lactones) are very reactive, making their isolation difficult. Special methods are normally required for the laboratory synthesis of small-ring lactones as well as those that contain rings larger than six-membered. Nomenclature Lactones are usually named according to the precursor acid molecule (''aceto'' = 2 carbon atoms, ''propio'' = 3, ''butyro'' = 4, ''valero'' = 5, ''capro'' = 6, etc.), with a ''-lactone'' suffix and a Greek alphabet, Greek letter prefix that specifies the number of carbon atoms in the heterocycle — that is, the distance between t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
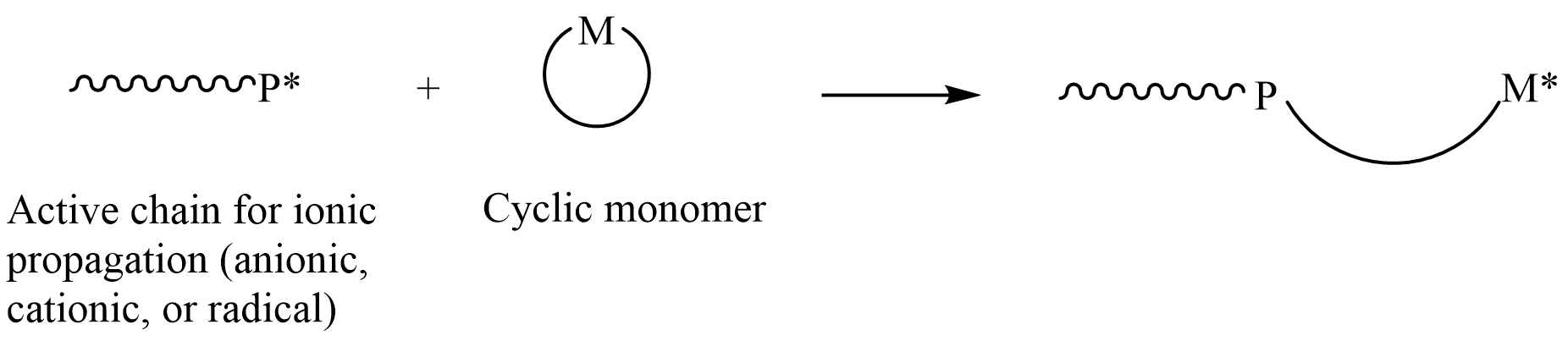
Ring Opening Polymerization
In polymer chemistry, ring-opening polymerization (ROP) is a form of chain-growth polymerization, in which the terminus of a polymer chain attacks cyclic monomers to form a longer polymer (see figure). The reactive center can be radical, anionic or cationic. Some cyclic monomers such as norbornene or cyclooctadiene can be polymerized to high molecular weight polymers by using metal catalysts. ROP is a versatile method for the synthesis of biopolymers. Ring-opening of cyclic monomers is often driven by the relief of bond-angle strain. Thus, as is the case for other types of polymerization, the enthalpy change in ring-opening is negative. Monomers Cyclic monomers that are amenable to ROP include epoxides, cyclic trisiloxanes, some lactones, lactides, cyclic carbonates, and amino acid N-carboxyanhydrides. Many strained cycloalkenes, e.g norbornene, are suitable monomers via ring-opening metathesis polymerization. History Ring-opening polymerization has been used since th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands. Alkoxides, although generally not stable in protic solvents such as water, occur widely as intermediates in various reactions, including the Williamson ether synthesis. Transition metal alkoxides are widely used for coatings and as catalysts. Enolates are unsaturated alkoxides derived by deprotonation of a bond adjacent to a ketone or aldehyde. The nucleophilic center for simple alkoxides is located on the oxygen, whereas the nucleophilic site on enolates is delocalized onto both carbon and oxygen sites. Ynolates are also unsaturated alkoxides derived from acetylenic alcohols. Phenoxides are close relatives of the alkoxides, in which the alkyl group is replaced by a derivative of be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tishchenko Reaction
The Tishchenko reaction is an organic chemical reaction that involves disproportionation of an aldehyde in the presence of an alkoxide. The reaction is named after Russian organic chemist Vyacheslav Tishchenko, who discovered that aluminium alkoxides are effective catalysts for the reaction. In the related Cannizzaro reaction, the base is sodium hydroxide and then the oxidation product is a carboxylic acid and the reduction product is an alcohol. History The reaction involving benzaldehyde was discovered by Claisen using sodium benzylate as base. The reaction produces benzyl benzoate. Enolizable aldehydes are not amenable to Claisen's conditions. Vyacheslav Tishchenko discovered that aluminium alkoxides allowed the conversion of enolizable aldehydes to esters. Examples * The Tishchenko reaction of acetaldehyde gives the commercially important solvent ethyl acetate. The reaction is catalyzed by aluminium alkoxides. * The Tishchenko reaction is used to obtain isobu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oppenauer Oxidation
Oppenauer oxidation, named after , is a gentle method for selectively oxidizing secondary alcohols to ketones. The reaction is the opposite Meerwein–Ponndorf–Verley reduction. The alcohol is oxidized with aluminium isopropoxide in excess acetone. This shifts the equilibrium toward the product side. The oxidation is highly selective for secondary alcohols and does not oxidize other sensitive functional groups such as amines and sulfides. Though primary alcohols can be oxidized under Oppenauer conditions, primary alcohols are seldom oxidized by this method due to the competing aldol condensation of aldehyde products. The Oppenauer oxidation is still used for the oxidation of acid labile substrates. The method has been largely displaced by oxidation methods based on chromates (e.g. pyridinium chlorochromate) or dimethyl sulfoxide (e.g. Swern oxidation) or Dess–Martin oxidation due to its use of relatively mild and non-toxic reagents (e.g. the reaction is run in acetone/benz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehydes
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are common and play important roles in the technology and biological spheres. Structure and bonding Aldehydes feature a carbon center that is connected by a double bond to oxygen and a single bond to hydrogen and single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2- hybridized. The aldehyde group is somewhat polar. The C=O bond length is about 120-122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes are more soluble in water, formaldehyde and acetaldehyde completely so. The volatile aldehydes have pungent odors. Aldehy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |