|
(S)-iPr-PHOX
(''S'')-iPr-PHOX, or (''S'')-2- -(diphenylphosphino)phenyl4-isopropyl-4,5-dihydrooxazole, is a chiral, bidentate, ligand derived from the amino alcohol valinol. It is part of a broader class of phosphinooxazolines ligands and has found application in asymmetric catalysis. Preparation (''S'')-iPr-PHOX is prepared using the amino alcohol valinol, which is derived from valine. The phosphine Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ... moiety may be introduced first, by a reaction between 2-bromobenzonitrile and chlorodiphenylphosphine; the oxazoline ring is then formed in a Witte Seeliger reaction. This yields an air stable zinc complex which must be treated with bipyridine in order to obtain the free ligand. Synthesis is performed under argon or nitrogen to avoid contact w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valinol
Valinol is an organic compound named after, and commonly produced from, the amino acid valine. The compound is chiral and is produced almost exclusively as the S‑isomer (also designated as the L‑isomer), due to the abundant supply of S-valine. It is part of a broader class of amino alcohols. Synthesis Valinol can be generated by converting the carboxylic group of valine to an alcohol with a strong reducing agent such as lithium aluminium hydride, or with NaBH4 and I2 (forming the borane–tetrahydrofuran complex). In both cases the valinol produced can be subsequently purified by short path distillation. Reactions Valinol is mainly used to prepare chiral oxazolines, a process which can be achieved via a variety of methods. These oxazolines are principally used as ligands in asymmetric catalysis Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphinooxazolines
Phosphinooxazolines (often abbreviated PHOX) are a class of chiral ligands used in asymmetric catalysis. Their complexes are particularly effective at generating single enatiomers in reactions involving highly symmetric transition states, such as allylic substitutions, which are typically difficult to perform stereoselectively. The ligands are bidentate and have been shown to be hemilabile with the softer P‑donor being more firmly bound than the harder N‑donor. Synthesis The synthesis of phosphinooxazolines is modular and it is not normally necessary to introduce the phosphine and oxazoline moieties in any particular order. However while examples exist of the phosphine being introduced first, it is more common to see the synthesis of a phenyloxazoline which is subsequently combined with a source of diphenylphosphine. Methods for doing this depend on the nature of the substituent in the X position: * When X = fluorine coupling involves anionic displacement with a dipheny ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxazoline
Oxazoline is a five-membered heterocyclic organic compound with the formula . It is the parent of a family of compounds called oxazolines (emphasis on plural), which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the unsaturated analogues of oxazolidines, and they are isomeric with isoxazolines, where the N and O are directly bonded. Two isomers of oxazoline are known, depending on the location of the double bond. Oxazoline itself has no applications however oxazolines have been widely investigated for potential applications. These applications include use as ligands in asymmetric catalysis, as protecting groups for carboxylic acids and increasingly as monomers for the production of polymers. Isomers Synthesis The synthesis of 2-oxazoline rings is well established and in general proceeds via the cyclisation of a 2-amino alcohol (typically obtained by the reduction of an amino acid) with a suitable functional group. The overall mechanism is usuall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chirality (chemistry)
In chemistry, a molecule or ion is called chiral () if it cannot be superposed on its mirror image by any combination of rotation (geometry), rotations, translation (geometry), translations, and some Conformational isomerism, conformational changes. This geometric property is called chirality (). The terms are derived from Ancient Greek χείρ (''cheir'') 'hand'; which is the canonical example of an object with this property. A chiral molecule or ion exists in two stereoisomers that are mirror images of each other, called enantiomers; they are often distinguished as either "right-handed" or "left-handed" by their absolute configuration or some other criterion. The two enantiomers have the same chemical properties, except when reacting with other chiral compounds. They also have the same physics, physical properties, except that they often have opposite optical activity, optical activities. A homogeneous mixture of the two enantiomers in equal parts is said to be racemic mixtu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Denticity
In coordination chemistry, denticity () refers to the number of donor groups in a given ligand that bind to the central metal atom in a coordination complex. In many cases, only one atom in the ligand binds to the metal, so the denticity equals one, and the ligand is said to be monodentate (sometimes called unidentate). Ligands with more than one bonded atom are called polydentate or multidentate. The denticity of a ligand is described with the Greek letter κ ('kappa'). For example, κ6-EDTA describes an EDTA ligand that coordinates through 6 non-contiguous atoms. Denticity is different from hapticity because hapticity refers exclusively to ligands where the coordinating atoms are contiguous. In these cases the η ('eta') notation is used. Bridging ligands use the μ ('mu') notation. Classes Polydentate ligands are chelating agents and classified by their denticity. Some atoms cannot form the maximum possible number of bonds a ligand could make. In that case one or mor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environmental chemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enantioselective Synthesis
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric (enantiomeric or diastereomeric) products in unequal amounts." Put more simply: it is the synthesis of a compound by a method that favors the formation of a specific enantiomer or diastereomer. Enantiomers are stereoisomers that have opposite configurations at every chiral center. Diastereomers are stereoisomers that differ at one or more chiral centers. Enantioselective synthesis is a key process in modern chemistry and is particularly important in the field of pharmaceuticals, as the different enantiomers or diastereomers of a molecule often have different biological activity. Overview Many of the building blocks of biological systems such as sugars and amino acids are produced exclusively as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valine
Valine (symbol Val or V) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α- carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain isopropyl group, making it a non-polar aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Human dietary sources are foods that contain protein, such as meats, dairy products, soy products, beans and legumes. It is encoded by all codons starting with GU (GUU, GUC, GUA, and GUG). History and etymology Valine was first isolated from casein in 1901 by Hermann Emil Fischer. The name valine comes from valeric acid, which in turn is named after the plant valerian due to the presence of the acid in the roots of the plant. Nomenclature According to IUPAC, carbon atoms forming valine are numbered sequentially s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting fish, due to the presence of substituted phosphine and diphosphane (). With traces of present, is spontaneously flammable in air ( pyrophoric), burning with a luminous flame. Phosphine is a highly toxic respiratory poison, and is immediately dangerous to life or health at 50 ppm. Phosphine has a trigonal pyramidal structure. Phosphines are compounds that include and the organophosphines, which are derived from by substituting one or more hydrogen atoms with organic groups. They have the general formula . Phosphanes are saturated phosphorus hydrides of the form , such as triphosphane. Phosphine, PH3, is the smallest of the phosphines and the smallest of the phosphanes. History Philippe Gengembre (1764–1838), a student of Lavois ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorodiphenylphosphine
Chlorodiphenylphosphine is an organophosphorus compound with the formula (C6H5)2PCl, abbreviated Ph2PCl. It is a colourless oily liquid with a pungent odor that is often described as being garlic-like and detectable even in the ppb range. It is useful reagent for introducing the Ph2P group into molecules, which includes many ligands.Quin, L. D. ''A Guide to Organophosphorus Chemistry''; Wiley IEEE: New York, 2000; pp 44-69. Like other halophosphines, Ph2PCl is reactive with many nucleophiles such as water and easily oxidized even by air. Synthesis and reactions Chlorodiphenylphosphine is produced on a commercial scale from benzene and phosphorus trichloride (PCl3). Benzene reacts with phosphorus trichloride at extreme temperatures around 600 °C to give dichlorophenylphosphine (PhPCl2) and HCl. Redistribution of PhPCl2 in the gas phase at high temperatures results in chlorodiphenylphosphine. :2PhPCl2 → Ph2PCl + PCl3 Alternatively such compounds are prepared by redistribu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
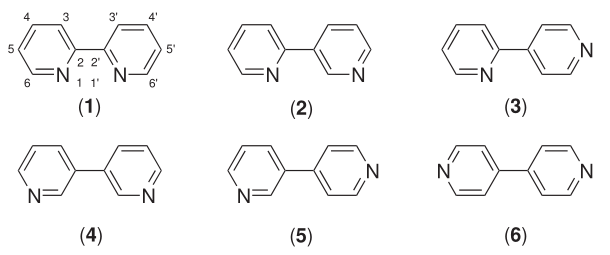
Bipyridine
Bipyridines also known as bipyridyls, dipyridyls, and dipyridines, are a family of chemical compounds with the formula (C5H4N)2, consisting of two pyridyl (C5H4N) rings. Pyridine is an aromatic nitrogen-containing heterocycle. Bipyridines are of significance in pesticides. Six isomers of bipyridine exist, but two are prominent: 2,2′-bipyridine is a popular ligand. 4,4'-Bipyridine is a precursor to the commercial herbicide paraquat. The bipyridines are all colourless solids, which are soluble in organic solvents and slightly soluble in water. 2,2′-Bipyridine 2,2′-Bipyridine (2,2′-bipy) is a chelating ligand that forms complexes with most transition metal ions that are of broad academic interest. Many of these complexes have distinctive optical properties, and some are of interest for analysis. Its complexes are used in studies of electron and energy transfer, supramolecular and materials chemistry, and catalysis. 2,2′-Bipyridine is used in the manufacture of diqua ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




4-3D-balls.png)