|
Nitroxide
Aminoxyl denotes a radical functional group with general structure R2N–O•. It is commonly known as a nitroxyl radical or a nitroxide, however IUPAC discourages the use of these terms, as they erroneously suggest the presence of a nitro group. Aminoxyls are structurally related to hydroxylamines and ''N''-oxoammonium salts, with which they can interconvert via a series of redox steps. Aminoxyl radical.svg, The general structure of the aminoxyl radical 2,2,6,6-Tetramethylpiperidinyloxyl.svg, TEMPO, a commonly encountered organic aminoxyl radical Kaliumnitrosodisulfonat.svg, Fremy's salt, an inorganic aminoxyl radical Sterically unhindered aminoxyls baring α-hydrogens are unstable and undergo rapid disproportionation to nitrones and hydroxylamines. Sterically hindered aminoxyls without α-hydrogens, such TEMPO and TEMPOL, and are persistent (stable) radicals and find use in a range of applications, both on the laboratory scale and in industry. Their ability to reversibly b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitroxide-mediated Radical Polymerization
Nitroxide-mediated radical polymerization is a method of radical polymerization that makes use of an nitroxide initiator to generate polymers with well controlled stereochemistry and a very low dispersity. It is a type of reversible-deactivation radical polymerization. Alkoxyamine Initiators The initiating materials for nitroxide-mediated radical polymerization (NMP) are a family of compounds referred to as alkoxyamines. An alkoxyamine can essentially be viewed as an alcohol bound to a secondary amine by an N-O single bond. The utility of this functional group is that under certain conditions, homolysis of the C-O bond can occur, yielding a stable radical in the form of a 2-center 3-electron N-O system and a carbon radical which serves as an initiator for radical polymerization. For the purposes of NMP, the R groups attached to the nitrogen are always bulky, sterically hindering groups and the R group in the O- position forms a stable radical, generally is benzylic for polymeriz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
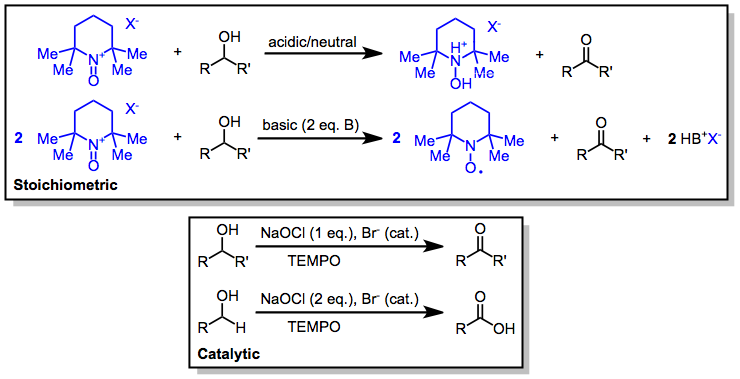
Oxoammonium-catalyzed Oxidation
Oxoammonium-catalyzed oxidation reactions involve the conversion of organic substrates to more highly oxidized materials through the action of an N-oxoammonium species. Nitroxides may also be used in catalytic amounts in the presence of a stoichiometric amount of a terminal oxidant. Bobbitt, J. M.; Bruckner, C.; Merbouh, N. '' Org. React.'' 2009, ''74'', 103. Nitroxide radical species used are either 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) or derivatives thereof. Mechanism and stereochemistry One-electron oxidation of the nitroxide produces a highly electrophilic oxoammonium species, which serves as the active oxidizing agent. The nitroxide can be used as a catalyst in conjunction with cheaper stoichiometric oxidants such as sodium hypochlorite or bis(acetoxy)iodobenzene (BAIB). Under neutral or slightly acidic conditions (in the presence of silica gel, for instance), oxidation occurs by an initial hydrogen bond between the hydroxyl group and the oxoammonium nitrogen, fol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-Oxoammonium Salt
''N''-Oxoammonium salts are a class of organic compounds with the formula 1R2=O−. The cation 1R2=Ois of interest for the dehydrogenation of alcohols. Oxoammonium salts are diamagnetic, whereas the nitroxide has a doublet ground state. A prominent nitroxide is prepared by oxidation of (2,2,6,6-tetramethylpiperidin-1-yl)oxyl, commonly referred to as EMPOsup>+. A less expensive analogue is Bobbitt's salt. Structure and bonding Oxoammonium cations are isoelectronic with carbonyls and structurally related to aldoximes (hydroxylamines), and aminoxyl (nitroxide) radicals, with which they can interconvert via a series of redox steps. According to X-ray crystallography, the N–O distance in EMPOF4 is 1.184 Å, 0.1 Å shorter than the N–O distance of 1.284 Å in the charge-neutral TEMPO. Similarly, the N in EMPOsup>+ is nearly planar, but the O moves 0.1765 Å out of the plane in the neutral TEMPO. The ''N''-oxoammonium salts are used for oxidation of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TEMPOL
4-Hydroxy-TEMPO or TEMPOL, formally 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl, is a heterocyclic compound. Like the related TEMPO, it is used as a catalyst and chemical oxidant by virtue of being a stable aminoxyl radical. Its major appeal over TEMPO is that is less expensive, being produced from triacetone amine, which is itself made via the condensation of acetone and ammonia. This makes it economically viable on an industrial scale. In biochemical research, 4-hydroxy-TEMPO has been investigated as an agent for limiting reactive oxygen species. It catalyzes the disproportionation of superoxide, facilitates hydrogen peroxide metabolism, and inhibits Fenton's reagent, Fenton chemistry. 4-Hydroxy-TEMPO, along with related nitroxides, are being studied for their potential antioxidant properties. On an industrial-scale 4-hydroxy-TEMPO is often present as a structural element in hindered amine light stabilizers, which are commonly used stabilizers in plastics, it is also used a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radical (chemistry)
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes. A notable example of a radical is the hydroxyl radical (HO·), a molecule that has one unpaired electron on the oxygen atom. Two other examples are triplet oxygen and triplet carbene (꞉) which have two unpaired electrons. Radicals may be generated in a number of ways, but typical methods involve redox reactions. Ionizing radiation, heat, electrical discharges, and electrolysis are known to produce radicals. Radicals are intermediates in many chemical reactions, more so than is apparent from the balanced equations. Radicals are important in combustion, atmospheric chemistry, polymerization, plasma chemistry, biochemistry, and many other chemical processes. A majority of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrone
In organic chemistry, a nitrone is a functional group consisting of an ''N''-oxide of an imine. The general structure is , where R’ is not a hydrogen. A nitrone is a 1,3-dipole, and is used in 1,3-dipolar cycloadditions. Other reactions of nitrones are known, including formal +3cycloadditions to form 6-membered rings, as well as formal +2cycloadditions to form 7-membered rings. Generation of nitrones Nitrones are generated most often either by the oxidation of hydroxylamines or condensation of monosubstituted hydroxylamines with carbonyl compounds (ketones or aldehydes). The most general reagent used for the oxidation of hydroxylamines is mercury(II) oxide. Carbonyl condensation methods avoid issues of site selectivity associated with the oxidation of hydroxylamines with two sets of (alpha) hydrogens. A significant problem associated with many reactive nitrones is dimerization. This issue is alleviated experimentally by employing an excess of the nitrone or increasing t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-Hydroxyphthalimide
''N''-Hydroxyphthalimide is the ''N''-hydroxy derivative of phthalimide. The compound can be utilized as a catalyst for oxidation reactions, in particular for the selective oxidation (e. g. alkanes to alcohols) with molecular oxygen under mild conditions. Occurrence and production The synthesis of ''N''-hydroxyphthalimide from phthaloyl chloride and hydroxylamine hydrochloride in the presence of sodium carbonate in aqueous solution was first reported by Lassar Cohn in 1880 (referred to as "Phthalylhydroxylamin"). The product forms as a red sodium salt under basic conditions, while white ''N''-hydroxyphthalimide precipitates in 55% yield as the solution is acidified. ''N''-hydroxyphthalimide is also produced by reacting hydroxylamine hydrochloride with diethyl phthalate in the presence of sodium acetate, or with phthalic anhydride in the presence of sodium carbonate with heating. In the last case, an overall yield of 76% is produced following purification by recrystallizati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymerization Inhibitor
Polymerisation inhibitors (US: polymerization inhibitors) are chemical compounds added to monomers to prevent their auto-polymerisation. Unsaturated monomers such as acrylates, vinyl chloride, butadiene and styrene require inhibitors for both processing and safe transport and storage. Many monomers are purified industrially by distillation, which can lead to thermally initiated polymerisation. Styrene for example is distilled at temperatures above 100 °C whereupon it undergoes thermal polymerisation at a rate of ~2% per hour. This polymerisation is undesirable, as it can foul the fractionating tower, it is also typically exothermic which can lead to a runaway reaction and potential explosion if left unchecked. Once initiated polymerisation is typically radical in mechanism and as such many polymerisation inhibitors act as radical scavengers. Inhibitors vs retarders The term 'inhibitor' is often used in a general sense to describe any compound used to prevent unwanted polymeri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antiozonant
An antiozonant, also known as anti-ozonant, is an organic compound that prevents or retards damage caused by ozone. The most important antiozonants are those which prevent degradation of elastomers like rubber. A number of research projects study the application of another type of antiozonats to protect plants. Effect of ozone Many elastomers are rich in unsaturated double bonds, which can react with ozone present in the air in process known as ozonolysis. This reaction breaks the polymer chains, degrading the mechanical properties of the material. The most obvious effect of this is cracking of the elastomer (ozone cracking), which is exacerbated by mechanical stress. The rate of degradation is effected both by the chemical structure of the elastomer and the amount of ozone in the environment. Elastomers which are rich in double bonds, such as natural rubber, polybutadiene, styrene-butadiene rubber and nitrile rubber are the most sensitive to degradation, whereas butyl rubbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
P-Phenylenediamine
''p''-Phenylenediamine (PPD) is an organic compound with the formula C6H4(NH2)2. This derivative of aniline is a white solid, but samples can darken due to air oxidation. It is mainly used as a component of engineering polymers and composites like kevlar. It is also an ingredient in hair dyes and is occasionally used as a substitute for henna. Production PPD is produced via three routes. Most commonly, 4-nitrochlorobenzene is treated with ammonia and the resulting 4-nitroaniline is then hydrogenated: :ClC6H4NO2 + 2 NH3 → H2NC6H4NO2 + NH4Cl :H2NC6H4NO2 + 3 H2 → H2NC6H4NH2 + 2 H2O In the DuPont route, aniline is converted to diphenyltriazine, which is then converted by acid-catalysis to 4-aminoazobenzene. Hydrogenation of the latter affords PPD.Robert A. Smiley "Phenylene- and Toluenediamines" in ''Ullmann's Encyclopedia of Industrial Chemistry'', 2002, Wiley-VCH, Weinheim. Uses Precursor to polymers PPD is a precursor to aramid plastics and fibers such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hindered Amine Light Stabilizers
Hindered amine light stabilizers (HALS) are chemical compounds containing an amine functional group that are used as stabilizers in plastics and polymers. These compounds are typically derivatives of tetramethylpiperidine and are primarily used to protect the polymers from the effects of photo-oxidation; as opposed to other forms of polymer degradation such as ozonolysis. They are also increasingly being used as thermal stabilizers, particularly for low and moderate level of heat, however during the high temperature processing of polymers (e.g. injection moulding) they remain less effective than traditional phenolic antioxidants. Mechanism of action HALS do not absorb UV radiation, but act to inhibit degradation of the polymer by continuously and cyclically removing free radicals that are produced by photo-oxidation of the polymer. The overall process is sometimes referred to as the Denisov cycle, after Evguenii T. Denisov and is exceedingly complex. Broadly, HALS react with th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer Stabilizers
Polymer stabilizers (British: polymer stabilisers) are chemical additives which may be added to polymeric materials, such as plastics and rubbers, to inhibit or retard their degradation. Common polymer degradation processes include oxidation, UV degradation, UV-damage, Thermal degradation of polymers, thermal degradation, ozonolysis, combinations thereof such as photo-oxidation of polymers, photo-oxidation, as well as reactions with catalyst residues, dyes, or impurities. All of these degrade the polymer at a chemical level, via chain scission, uncontrolled recombination and cross-linking, which adversely affects many key properties such as strength, malleability, appearance and colour. Stabilizers are used at all stages of the polymer life-cycle. They allow plastic items to be produced faster and with fewer defects, extend their useful lifespan, and facilitate their recycling. However they also continue to stabilise waste plastic, causing it to remain in the environment for longer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |