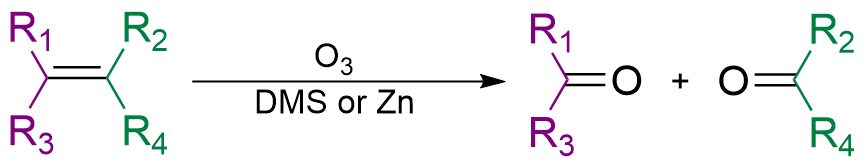|
Antiozonant
An antiozonant, also known as anti-ozonant, is an organic compound that prevents or retards damage caused by ozone. The most important antiozonants are those which prevent degradation of elastomers like rubber. A number of research projects study the application of another type of antiozonats to protect plants. Effect of ozone Many elastomers are rich in unsaturated double bonds, which can react with ozone present in the air in process known as ozonolysis. This reaction breaks the polymer chains, degrading the mechanical properties of the material. The most obvious effect of this is cracking of the elastomer (ozone cracking), which is exacerbated by mechanical stress. The rate of degradation is effected both by the chemical structure of the elastomer and the amount of ozone in the environment. Elastomers which are rich in double bonds, such as natural rubber, polybutadiene, styrene-butadiene rubber and nitrile rubber are the most sensitive to degradation, whereas butyl rubbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
6PPD
6PPD is an organic chemical widely used as stabilising additive (or antidegradant) in rubbers, such as NR, SBR and BR; all of which are common in vehicle tires. Although it is an effective antioxidant it is primarily used because of its excellent antiozonant peformance. It is one of several antiozonants based around ''p''-phenylenediamine (PPD). Manufacturing 6PPD is prepared by reductive amination of methyl isobutyl ketone with 4-aminodiphenylamine. This produces a racemic mixture. Application 6PPD is a common rubber antiozonant, with a major application in vehicle tires. It is mobile within the rubber and is slowly forced to the surface via blooming. Here it forms a "scavenger-protective film", reacting with the ozone more quickly that the ozone can react with the rubber. This process initially forms aminoxyl radicals and was thought to stop at the quinone diimine, but is now known to continue to form quinones, amongst other products. Despite 6PPD being used in tires sinc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-Isopropyl-N'-phenyl-1,4-phenylenediamine
''N''-Isopropyl-''N''′-phenyl-1,4-phenylenediamine (often abbreviated IPPD) is an organic compound commonly used as an antiozonant in rubbers, particularly those used for tires. Like other p-phenylenediamine-based antiozonants it works by virtue of its low ionization energy, which allows it to react with ozone faster than ozone will react with rubber. This reaction converts it to the corresponding aminoxyl radical (R2N–O•), with the ozone being converted to a hydroperoxyl radical (HOO•), these species can then be scavenged by other antioxidant polymer stabilizers. IPPD is prone to process called blooming, where it migrates to the surface of the rubber. This can be beneficial to the tire, as ozone attacks the tire surface and blooming therefore moves the antiozonant to where it is most needed, however this also increases the leaching of IPPD into the environment. Many tire producers have moved to using 6PPD instead, as this migrates more slowly. Oxidation of IPPD convert ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ozone Cracking
Cracks can be formed in many different elastomers by ozone attack, and the characteristic form of attack of vulnerable rubbers is known as ozone cracking. The problem was formerly very common, especially in tires, but is now rarely seen in those products owing to preventive measures. However, it does occur in many other safety-critical items such as fuel lines and rubber seals, such as gaskets and O-rings, where ozone attack is considered unlikely. Only a trace amount of the gas is needed to initiate cracking, and so these items can also succumb to the problem. Susceptible elastomers Tiny traces of ozone in the air will attack double bonds in rubber chains, with natural rubber, polybutadiene, styrene-butadiene rubber and nitrile rubber being most sensitive to degradation. Every repeat unit in the first three materials has a double bond, so every unit can be degraded by ozone. Nitrile rubber is a copolymer of butadiene and acrylonitrile units, but the proportion of acrylonitrile ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene Diurea
Ethylene diurea (EDU) is an organic compound with the formula (CH2NHCONH2)2. It is a white solid. The compound has attracted interest as a potential antiozonant for crop protection. With respect to preventing the harmful effects on crops by ozone Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lo ..., EDU appears to either prevent the harmful effects of ozone or it stimulated plant growth. Trees treated with EDU were significantly healthier in both leaf longevity and water use efficiency. The effectiveness of EDU depends upon several environmental factors. References {{reflist Ureas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tire Manufacturing
Pneumatic tires are manufactured according to relatively standardized processes and machinery, in around 455 tire factories in the world. With over 1 billion tires manufactured worldwide annually, the tire industry is a major consumer of natural rubber. Tire factories start with bulk raw materials such as synthetic rubber (60% -70% of total rubber in the tire industry), carbon black, and chemicals and produce numerous specialized components that are assembled and cured. The tire is an assembly of numerous components that are built up on a drum and then cured in a press under heat and pressure. Heat facilitates a polymerization reaction that crosslinks rubber monomers to create long elastic molecules. Inner liner The inner liner is a calendered halobutyl rubber sheet compounded with additives that result in low air permeability. The inner liner assures that the tire will hold high-pressure air inside, without an inner tube, minimizing diffusion through the rubber structure. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tire Manufacturing
Pneumatic tires are manufactured according to relatively standardized processes and machinery, in around 455 tire factories in the world. With over 1 billion tires manufactured worldwide annually, the tire industry is a major consumer of natural rubber. Tire factories start with bulk raw materials such as synthetic rubber (60% -70% of total rubber in the tire industry), carbon black, and chemicals and produce numerous specialized components that are assembled and cured. The tire is an assembly of numerous components that are built up on a drum and then cured in a press under heat and pressure. Heat facilitates a polymerization reaction that crosslinks rubber monomers to create long elastic molecules. Inner liner The inner liner is a calendered halobutyl rubber sheet compounded with additives that result in low air permeability. The inner liner assures that the tire will hold high-pressure air inside, without an inner tube, minimizing diffusion through the rubber structure. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
P-Phenylenediamine
''p''-Phenylenediamine (PPD) is an organic compound with the formula C6H4(NH2)2. This derivative of aniline is a white solid, but samples can darken due to air oxidation. It is mainly used as a component of engineering polymers and composites like kevlar. It is also an ingredient in hair dyes and is occasionally used as a substitute for henna. Production PPD is produced via three routes. Most commonly, 4-nitrochlorobenzene is treated with ammonia and the resulting 4-nitroaniline is then hydrogenated: :ClC6H4NO2 + 2 NH3 → H2NC6H4NO2 + NH4Cl :H2NC6H4NO2 + 3 H2 → H2NC6H4NH2 + 2 H2O In the DuPont route, aniline is converted to diphenyltriazine, which is then converted by acid-catalysis to 4-aminoazobenzene. Hydrogenation of the latter affords PPD.Robert A. Smiley "Phenylene- and Toluenediamines" in ''Ullmann's Encyclopedia of Industrial Chemistry'', 2002, Wiley-VCH, Weinheim. Uses Precursor to polymers PPD is a precursor to aramid plastics and fibers such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ozonolysis
In organic chemistry, ozonolysis is an organic reaction where the unsaturated bonds of alkenes (), alkynes (), or azo compounds () are cleaved with ozone (). Alkenes and alkynes form organic compounds in which the multiple carbon–carbon bond has been replaced by a carbonyl () group while azo compounds form nitrosamines (). The outcome of the reaction depends on the type of multiple bond being oxidized and the work-up conditions. Ozonolysis of alkenes Alkenes can be oxidized with ozone to form alcohols, aldehydes or ketones, or carboxylic acids. In a typical procedure, ozone is bubbled through a solution of the alkene in methanol at −78 °C until the solution takes on a characteristic blue color, which is due to unreacted ozone. This indicates complete consumption of the alkene. Alternatively, various other chemicals can be used as indicators of this endpoint by detecting the presence of ozone. If ozonolysis is performed by bubbling a stream of ozone-enriched oxygen t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lower atmosphere to (dioxygen). Ozone is formed from dioxygen by the action of ultraviolet (UV) light and electrical discharges within the Earth's atmosphere. It is present in very low concentrations throughout the latter, with its highest concentration high in the ozone layer of the stratosphere, which absorbs most of the Sun's ultraviolet (UV) radiation. Ozone's odour is reminiscent of chlorine, and detectable by many people at concentrations of as little as in air. Ozone's O3 structure was determined in 1865. The molecule was later proven to have a bent structure and to be weakly diamagnetic. In standard conditions, ozone is a pale blue gas that condenses at cryogenic temperatures to a dark blue liquid and finally a violet-black soli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ground-level Ozone
Ground-level ozone (O3), also known as surface-level ozone and tropospheric ozone, is a trace gas in the troposphere (the lowest level of the Earth's atmosphere), with an average concentration of 20–30 parts per billion by volume (ppbv), with close to 100 ppbv in polluted areas. Ozone is also an important constituent of the stratosphere, where the ozone layer (2 to 8 parts per million ozone) exists which is located between 10 and 50 kilometers above the Earth's surface. The troposphere extends from the ground up to a variable height of approximately 14 kilometers above sea level. Ozone is least concentrated in the ground layer (or planetary boundary layer) of the troposphere. Ground-level or tropospheric ozone is created by chemical reactions between NOx gases (oxides of nitrogen produced by combustion) and volatile organic compounds (VOCs). The combination of these chemicals in the presence of sunlight form ozone. Its concentration increases as height above sea level increases, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paraffin Wax
Paraffin wax (or petroleum wax) is a soft colorless solid derived from petroleum, coal, or oil shale that consists of a mixture of hydrocarbon molecules containing between 20 and 40 carbon atoms. It is solid at room temperature and begins to melt above approximately , and its boiling point is above . Common applications for paraffin wax include lubrication, electrical insulation, and candles; dyed paraffin wax can be made into crayons. It is distinct from kerosene and other petroleum products that are sometimes called paraffin. Un-dyed, unscented paraffin candles are odorless and bluish-white. Paraffin wax was first created by Carl Reichenbach in Germany in 1830 and marked a major advancement in candlemaking technology, as it burned more cleanly and reliably than tallow candles and was cheaper to produce. In chemistry, ''paraffin'' is used synonymously with ''alkane'', indicating hydrocarbons with the general formula C''n''H2''n''+2. The name is derived from Latin ''parum'' (" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microcrystalline Wax
Microcrystalline waxes are a type of wax produced by de-oiling petrolatum, as part of the petroleum refining process. In contrast to the more familiar paraffin wax which contains mostly unbranched alkanes, microcrystalline wax contains a higher percentage of isoparaffinic (branched) hydrocarbons and naphthenic hydrocarbons. It is characterized by the fineness of its crystals in contrast to the larger crystal of paraffin wax. It consists of high molecular weight saturated aliphatic hydrocarbons. It is generally darker, more viscous, denser, tackier and more elastic than paraffin waxes, and has a higher molecular weight and melting point. The elastic and adhesive characteristics of microcrystalline waxes are related to the non-straight chain components which they contain. Typical microcrystalline wax crystal structure is small and thin, making them more flexible than paraffin wax. It is commonly used in cosmetic formulations. Microcrystalline waxes when produced by wax refiners are ty ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |