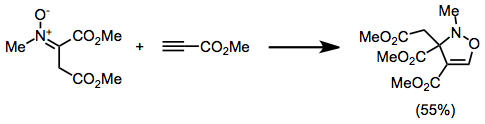|
Nitrone
In organic chemistry, a nitrone is a functional group consisting of an ''N''-oxide of an imine. The general structure is , where R’ is not a hydrogen. A nitrone is a 1,3-dipole, and is used in 1,3-dipolar cycloadditions. Other reactions of nitrones are known, including formal +3cycloadditions to form 6-membered rings, as well as formal +2cycloadditions to form 7-membered rings. Generation of nitrones Nitrones are generated most often either by the oxidation of hydroxylamines or condensation of monosubstituted hydroxylamines with carbonyl compounds (ketones or aldehydes). The most general reagent used for the oxidation of hydroxylamines is mercury(II) oxide. Carbonyl condensation methods avoid issues of site selectivity associated with the oxidation of hydroxylamines with two sets of (alpha) hydrogens. A significant problem associated with many reactive nitrones is dimerization. This issue is alleviated experimentally by employing an excess of the nitrone or increasing t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrone Cycloaddition
In organic chemistry, a nitrone is a functional group consisting of an ''N''-oxide of an imine. The general structure is , where R’ is not a hydrogen. A nitrone is a 1,3-dipole, and is used in 1,3-dipolar cycloadditions. Other reactions of nitrones are known, including formal +3cycloadditions to form 6-membered rings, as well as formal +2cycloadditions to form 7-membered rings. Generation of nitrones Nitrones are generated most often either by the oxidation of hydroxylamines or condensation of monosubstituted hydroxylamines with carbonyl compounds (ketones or aldehydes). The most general reagent used for the oxidation of hydroxylamines is mercury(II) oxide. Carbonyl condensation methods avoid issues of site selectivity associated with the oxidation of hydroxylamines with two sets of (alpha) hydrogens. A significant problem associated with many reactive nitrones is dimerization. This issue is alleviated experimentally by employing an excess of the nitrone or increasing th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxylamine
Hydroxylamine is an inorganic compound with the formula . The material is a white crystalline, hygroscopic compound.Greenwood and Earnshaw. ''Chemistry of the Elements.'' 2nd Edition. Reed Educational and Professional Publishing Ltd. pp. 431–432. 1997. Hydroxylamine is almost always provided and used as an aqueous solution. It is consumed almost exclusively to produce Nylon-6. It is also an intermediate in biological nitrification. The oxidation of to hydroxylamine is a step in biological nitrification. History Hydroxylamine was first prepared as hydroxylammonium chloride in 1865 by the German chemist Wilhelm Clemens Lossen (1838-1906); he reacted tin and hydrochloric acid in the presence of ethyl nitrate. It was first prepared in pure form in 1891 by the Dutch chemist Lobry de Bruyn and by the French chemist Léon Maurice Crismer (1858-1944). The coordination complex , known as Crismer's salt, releases hydroxylamine upon heating. Production Hydroxylamine or its salts can be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoxazolidine
An oxazolidine is a five-membered ring compound consisting of three carbon atoms, a nitrogen atom and an oxygen atom. The O atom and NH group are the 1 and 3 positions, respectively. In oxazolidine derivatives, there is always a carbon atom between the O and N atoms (or it would be an ''isoxazolidine''). All of the carbon atoms in oxazolidines are reduced (compare to oxazole and oxazoline). Some of their derivatives, the 2,4-Oxazolidinedione, oxazolidinediones, are used as anticonvulsants. Oxazolidines were first synthesized over 100 years ago. Monooxazolidines Oxazolidines that are the precursor to bisoxazolidines are in effect mono-oxazolidines. They are also used as moisture scavengers in polyurethane and other systems. Dioxooxazolidines Oxazolidines where the carbon centers at the 1 and 3 positions are carbonyl group, carbonyls are called dioxooxazolidines. Some of these are commercial fungicides including chlozolinate, vinclozolin, and famoxadone. Bisoxazolidines Bisoxazol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,3-dipole
In organic chemistry, a 1,3-dipolar compound or 1,3-dipole is a dipolar compound with delocalized electrons and a separation of charge over three atoms. They are reactants in 1,3-dipolar cycloadditions. The dipole has at least one resonance structure with positive and negative charges having a 1,3 relationship which can generally be denoted as , where a may be a carbon, oxygen or nitrogen, b may be nitrogen or oxygen, and c may be a carbon, oxygen or nitrogen. Known 1,3-dipoles are: * Azides () * Ozone () * Nitro compounds () * Diazo compounds () * Some oxides ** Azoxide compounds (RN(O)NR) ** Carbonyl oxides ( Criegee zwitterions)Li, Jie Jack''Criegee mechanism of ozonolysis''Book: Name Reactions. 2006, 173-174, ** Nitrile oxides () ** Nitrous oxide () ** Nitrones () * Some imines: ** Azomethine imine ** Nitrilimines (, analogous to nitrile oxide) ** Carbonyl imines * Some ylides ** Azomethine ylide ** Nitrile ylide Nitrile ylides also known as ''nitrilium ylides'', or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the Multiplicity (chemistry)#Molecules, bond multiplicity". The resulting reaction is a cyclization reaction. Many but not all cycloadditions are Concerted reaction, concerted and thus pericyclic. Nonconcerted cycloadditions are not pericyclic. As a class of addition reaction, cycloadditions permit carbon–carbon bond formation without the use of a nucleophile or electrophile. Cycloadditions can be described using two systems of notation. An older but still common notation is based on the size of linear arrangements of atoms in the reactants. It uses parentheses: where the variables are the numbers of linear atoms in each reactant. The product is a cycle of size . In this system, the standard Diels-Alder reaction is a (4 + 2)-cyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-Oxoammonium Salt
''N''-Oxoammonium salts are a class of organic compounds with the formula 1R2=O−. The cation 1R2=Ois of interest for the dehydrogenation of alcohols. Oxoammonium salts are diamagnetic, whereas the nitroxide has a doublet ground state. A prominent nitroxide is prepared by oxidation of (2,2,6,6-tetramethylpiperidin-1-yl)oxyl, commonly referred to as EMPOsup>+. A less expensive analogue is Bobbitt's salt. Structure and bonding Oxoammonium cations are isoelectronic with carbonyls and structurally related to aldoximes (hydroxylamines), and aminoxyl (nitroxide) radicals, with which they can interconvert via a series of redox steps. According to X-ray crystallography, the N–O distance in EMPOF4 is 1.184 Å, 0.1 Å shorter than the N–O distance of 1.284 Å in the charge-neutral TEMPO. Similarly, the N in EMPOsup>+ is nearly planar, but the O moves 0.1765 Å out of the plane in the neutral TEMPO. The ''N''-oxoammonium salts are used for oxidation of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chem
Chem may refer to: * Chemistry practical waali mam *Chemistry *Chemical * ''Chem'' (journal), a scientific journal published by Cell Press *Post apocalyptic slang for "drugs", medicinal or otherwise in the Fallout video game series. In Ancient Egyptian usage: * ''Khem'' (also spelt ''Chem''), the Egyptian word for "black" * Min (god), in the past erroneously named ''Khem'' CHEM may refer to : *A metabolic panel: for instance, CHEM-7, which is the basic metabolic panel *CHEM-DT CHEM-DT is the TVA owned-and-operated television station in Trois-Rivières, Quebec, Canada. It broadcasts a high-definition digital signal on VHF channel 8 from a transmitter on Rue Principale in Notre-Dame-du-Mont-Carmel. Owned by the Grou ..., a Canadian television channel See also * Chemo (other) * Kemi, a place in Finland {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ChemPlusChem
''ChemPlusChem'' is a monthly peer-reviewed scientific journal covering chemistry and published by Wiley-VCH on behalf of Chemistry Europe. It was established in 1929 by E. Votoček and J. Heyrovský and renamed in 1939 to ''Collection tschechischer chemischer Forschungsarbeiten/Collection des travaux chimiques tchèques/Collection of Czech Chemical Communications'' for one year. Publication was suspended until 1947, when it resumed publication as ''Collection of Czechoslovak Chemical Communications''. It obtained its current name in 2012. Abstracting and indexing The journal is abstracted and indexed in: * Chemical Abstracts Service * Chemistry Citation Index * Current Contents/Physical, Chemical & Earth Sciences * Science Citation Index Expanded * Scopus According to the ''Journal Citation Reports'', the journal has a 2021 impact factor of 3.210. References External links *{{Official website, https://chemistry-europe.onlinelibrary.wiley.com/journal/21926506 Chemistry Euro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |