1,3-dipole on:
[Wikipedia]
[Google]
[Amazon]
In 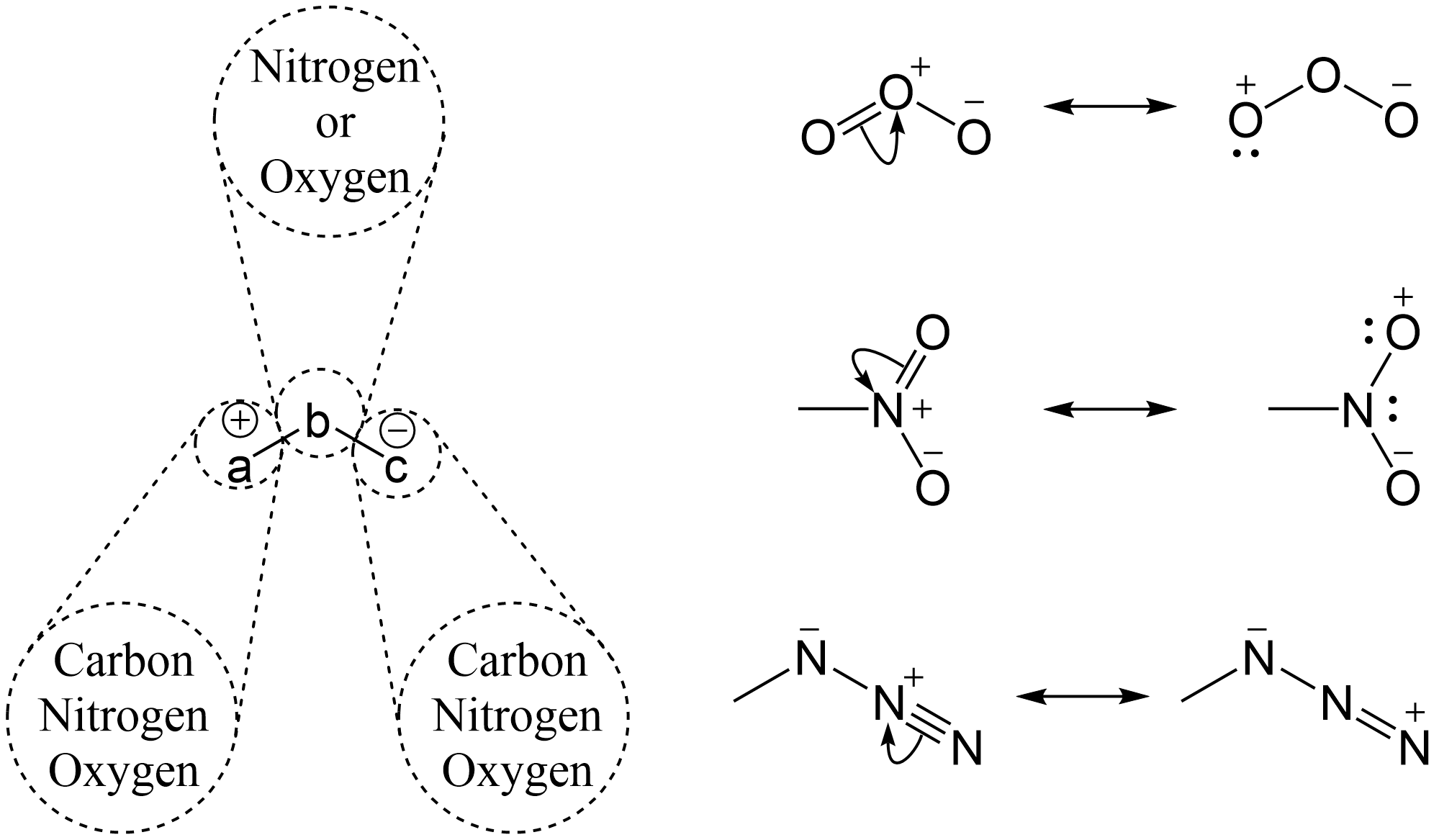 Known 1,3-dipoles are:
*
Known 1,3-dipoles are:
*
''Criegee mechanism of ozonolysis''
Book: Name Reactions. 2006, 173-174, ** * Some
* Some
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
, a 1,3-dipolar compound or 1,3-dipole is a dipolar compound
In organic chemistry, a dipolar compound or simply dipole is an electrically neutral molecule carrying a positive and a negative charge in at least one canonical description. In most dipolar compounds the charges are delocalized.
Unlike salts, dip ...
with delocalized electron
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly di ...
s and a separation of charge over three atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
s. They are reactants in 1,3-dipolar cycloadditions.
The dipole has at least one resonance structure
In chemistry, resonance, also called mesomerism, is a way of describing Chemical bond, bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance stru ...
with positive and negative charges having a 1,3 relationship which can generally be denoted as , where a may be a carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
, oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
or nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
, b may be nitrogen or oxygen, and c may be a carbon, oxygen or nitrogen.
 Known 1,3-dipoles are:
*
Known 1,3-dipoles are:
* Azide
In chemistry, azide is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant applic ...
s ()
* Ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lo ...
()
* Nitro compound
In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups (). The nitro group is one of the most common explosophores (functional group that makes a compound explosive) used globally. The nitr ...
s ()
* Diazo
The diazo group is an organic moiety consisting of two linked nitrogen atoms ( azo) at the terminal position. Overall charge neutral organic compounds containing the diazo group bound to a carbon atom are called diazo compounds or diazoalkanes ...
compounds ()
* Some oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the E ...
s
** Azoxide compounds (RN(O)NR)
** Carbonyl oxides ( Criegee zwitterions)Li, Jie Jack''Criegee mechanism of ozonolysis''
Book: Name Reactions. 2006, 173-174, **
Nitrile oxide
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix '' cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including met ...
s ()
** Nitrous oxide
Nitrous oxide (dinitrogen oxide or dinitrogen monoxide), commonly known as laughing gas, nitrous, or nos, is a chemical compound, an oxide of nitrogen with the formula . At room temperature, it is a colourless non-flammable gas, and has a ...
()
** Nitrones ()
imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bo ...
s:
** Azomethine imine
** Nitrilimine Nitrilimines or nitrile amides are a class of organic compounds sharing a common functional group with the general structure R-CN-NR corresponding to the conjugate base of an amine bonded to the N-terminus of a nitrile. The dominant structure for th ...
s (, analogous to nitrile oxide)
** Carbonyl imines
* Some ylide An ylide or ylid () is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms h ...
s
** Azomethine ylide
Azomethine ylides are nitrogen-based 1,3-dipoles, consisting of an iminium ion next to a carbanion. They are used in 1,3-dipolar cycloaddition reactions to form five-membered heterocycles, including pyrrolidines and pyrrolines. These reactions ...
** Nitrile ylide Nitrile ylides also known as ''nitrilium ylides'', or ''nitrilium methylides'' are generally reactive intermediates. With a few exceptions, they cannot be isolated. However, a structure has been determined on particularly stable nitrile ylideby X-r ...
()
** Carbonyl ylide
** Thiosulfines ()
References
{{Reflist Organic chemistry